Parvovirus B19 is a non-enveloped DNA virus that is transfusion transmissible1 and resistant to pathogen reduction processes in plasma product manufacturing. Parvovirus B19 is a common, acute, community-acquired infection, and most adults show evidence of prior exposure from natural infection. Transmission occurs mainly through respiratory secretions, but vertical transmission (mother-to-fetus) also occurs. Infections in the community may occur in periodic epidemics.1
FAQ: Parvovirus B19 Virus at Canadian Blood Services
Authors: Nicole Relke, MD, FRCPC; Mark Bigham, MD, MHSc, FRCPC; Aditi Khandelwal, MSc, MD, FRCPC, DRCPSC.
Publication date: October 3, 2025
Primary target audience: Transfusion medicine staff in hospitals
Background
Plasma fractionators report plasma nucleic acid test (NAT) results for parvovirus B19 to Canadian Blood Services within a timeframe that may incur the recall of in-date products. These test results may also result in follow up management by the hospital that received the blood component(s) and further management if it was transfused.
Frequently asked questions
1. What is parvovirus B19?
2. What are symptoms of parvovirus B19 infection?
Many cases of parvovirus B19 are asymptomatic; however, some people may experience a flu-like illness (i.e., fever, headache, chills, nausea, diarrhea, arthralgia) that resolves in 1-2 weeks. Classically, parvovirus B19 can cause erythema infectiosum (also known as fifths disease), characterized by a classic red rash involving the cheeks causing a “slapped cheek” appearance. This facial rash can be followed by a lace-like rash on the trunk and extremities. However, infected adults may not present with rash but may have polyarthropathy.
The time course of parvovirus B19 infection is shown in Figure 1.
In immunocompetent people, hematologic abnormalities (e.g., reticulocytopenia, anemia, leukopenia and/or thrombocytopenia) can be transiently observed. When monitoring blood counts, reticulocytopenia may occur as early as day 4 and reaches a nadir by day 8 and expected to resolve by day 18. After acquisition of parvovirus B19, viremia may occur as early as day 6, however symptoms may not develop in a small proportion of individuals until day 8 (see Figure 1). This means that a donor’s blood may be infectious if collected during the latter incubation period of an acute parvovirus B19 infection. This means, there is a 48-hour timeframe or longer where an individual may be an asymptomatic carrier. In contrast, immunocompromised patients have decreased ability to clear the virus and can have chronic infection.
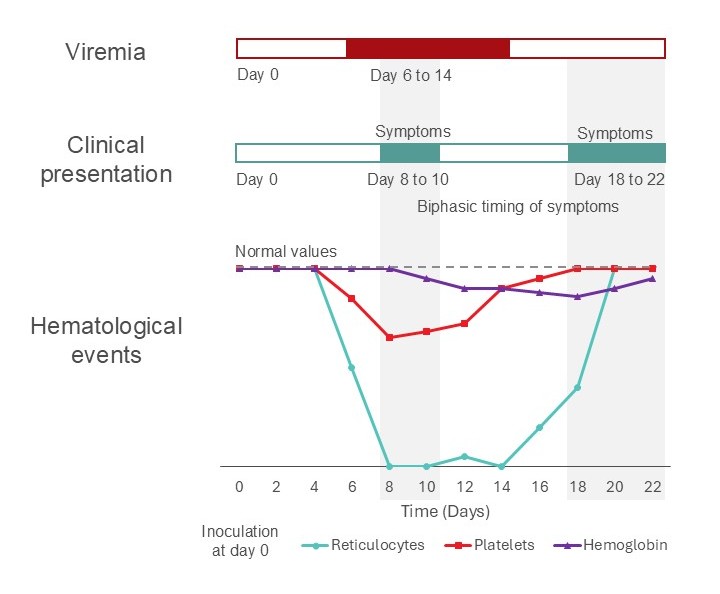
Figure 1. Time course for a typical case of parvovirus B19 illness in an immune competent adult. The hematological events highlight the departure from normal values for blood cells associated with parvovirus B19 viremia. Note: The duration of viremia in this figure is based on an individual who is immune competent; immunocompromised individuals may have prolonged viremia.
3. Are some people at higher risk for complications?
Yes, recipients with an increased risk for serious complications of parvovirus B19 infection include:
- People who are pregnant or a person of child-bearing age due to the risk of transmission to the fetus and complications such as fetal anemia and/or death, especially early in pregnancy.
- Immunocompromised patients including bone marrow or solid organ transplant recipients or those with an underlying hematologic disorder (i.e., risk of pure red cell aplasia or pancytopenia).
- Patients with an underlying hematological disorder associated with shortened red blood cell survival, including sickle cell, thalassemia and congenital hemolysis (i.e., risk of aplastic crisis).
4. What is the risk of parvovirus B19 transmission through blood?
Acute parvovirus B19 infection may be associated with high titer viremia approximately 1 week following exposure, lasting for ~5 days. Rare cases of transmission have been reported from blood or plasma-derived products, usually when viral DNA levels are ≥ 107 IU/mL. Pathogen-reduction technologies used for platelet and plasma products are less effective against parvovirus B19. However, the actual risk of viral transmission is unknown for a variety of reasons:
- Many transfusion recipients may be immune from prior infection.
- A significant risk of community exposure.
- Asymptomatic, clinically unrecognized infection.
5. When is donor plasma tested for parvovirus B19?
Canadian Blood Services does not currently test for parvovirus B19, as this is not required for release of fresh blood components in Canada under the Blood Regulations. However, since parvovirus B19 can escape pathogen-reduction steps, Health Canada requires that plasma fractionators test pooled plasma for B19 as a risk mitigation measure. Similarly, the U.S. FDA requires that donor plasma used for manufacturing contain ≤104 IU/mL of B19 DNA, since higher levels have been linked to transfusion transmission. Commercial plasma fractionators perform quality testing of plasma pools for parvovirus B19 using a nucleic acid test (NAT). Plasma fractionators report parvovirus B19 NAT results to Canadian Blood Services within a timeframe that may incur recall of in-date products.2
6. Why does Canadian Blood Services collect information on parvovirus B19 from the fractionator and what do they do with positive results?
As parvovirus B19 can be transmitted through blood products, fractionators notify Canadian Blood Services when a donor sample has a parvovirus B19 DNA titre >104 IU/mL. In such cases, Canadian Blood Services recalls all components collected within 3 weeks of an implicated donation (the inferred period of potential blood infectivity), notifies the hospital of the positive result, and tracks the epidemiology of parvovirus B19 in plasma donations.
7. I received a recall notice from Canadian Blood Services indicating that a donor has a positive nucleic acid test for parvovirus B19. What does this mean for a patient who received a potentially infectious blood transfusion?
Most adults in Canada have already been infected with parvovirus B19 and are protected from re-infection. However, a susceptible recipient of a potentially infectious blood transfusion could develop infection within days to a couple of weeks after transfusion.
If a patient has received a co-component (i.e., platelets, red blood cells) from a donor who later tested positive for parvovirus B19, we recommend a review of the patient’s chart to determine whether further investigation and notification of the clinical team is warranted. In some cases, discussion with the patient’s attending physician is appropriate. This recommendation applies to platelet products because current pathogen-reduction technologies used in manufacturing only partially reduce parvovirus B19 levels
If transfusion-transmitted infection is suspected, the recipient should be notified, especially if they are at risk of serious complications, as outlined in question 3. Recipient testing (complete blood count, reticulocyte count, parvovirus B19 DNA, IgG, IgM) may be performed at the discretion of the treating physician.
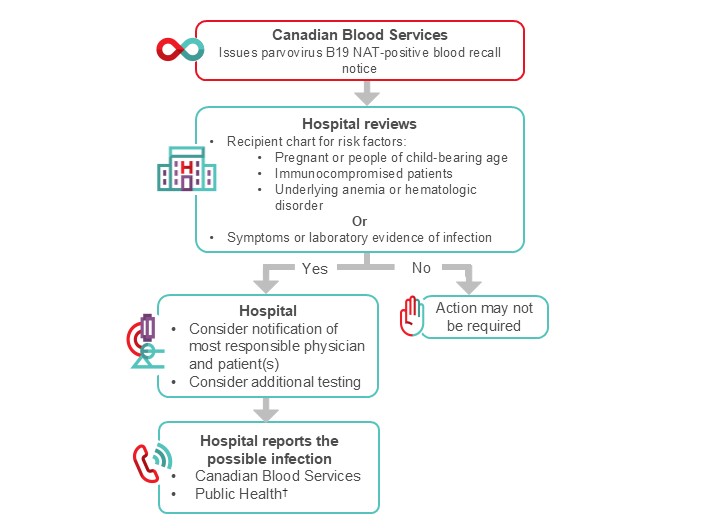
Figure 2. Suggested algorithm for hospital transfusion medicine staff for the management of parvovirus nucleic acid test (NAT) positive recall. † Where applicable, report possible parvovirus transfusion infection to Public Health.3
8. I assessed that a possible transfusion-transmitted parvovirus B19 infection occurred. What reporting is required?
Please report possible transfusion-transmitted parvovirus B19 infection to Canadian Blood Services. Parvovirus B19 is a reportable infection to some provincial Public Health authorities (e.g. Alberta, and Northwest Territories).3
Additional resources
AABB Parvovirus B19 Information:
- AABB page: https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/infectious-diseases/emerging-infectious-disease-agents
- Special issue of the journal, Transfusion, see Viral agents: https://onlinelibrary.wiley.com/toc/15372995/2024/64/S1
Suggested citation
Relke, N., Bigham, M. & Khandelwal, A. (2025, October 3) FAQ: Parvovirus B19 Virus at Canadian Blood Services. Canadian Blood Services. Retrieved Month day, year, from: https://profedu.blood.ca/en/transfusion/publications/faq-parvovirus-b19-virus-canadian-blood-services
References
- Viral agents (2nd section). (2024). Transfusion, 64(S1), S105-S107. https://doi.org/https://doi.org/10.1111/trf.17630
- Drews, S. J., Charlton, C., O'Brien, S. F., Burugu, S., & Denomme, G. A. (2024). Decreasing parvovirus B19 and hepatitis A nucleic acid test positivity rates in Canadian plasma donors following the initiation of COVID-19 restriction in March 2020. Vox Sang, 119(6), 624-629. https://doi.org/10.1111/vox.13616
- Territories, G. o. N. (2023). Parvovirus B19 (Fifth Disease). Retrieved from https://www.hss.gov.nt.ca/professionals/en/services/communicable-disease-manual/parvovirus-b19-fifth-disease?