FAQ: Information for health professionals ordering granulocyte concentrates
Authors: Jennifer Stepien, Marie-Hélène Robert
Primary target audiences: Transfusion medicine physicians, non-transfusion medicine physicians, nurses, medical laboratory technologists in a hospital laboratory
NOTE: This FAQ, developed in collaboration with Héma-Québec, provides information for granulocyte concentrate ordering, collection, delivery and reporting requirements. For additional information about granulocyte concentrates, see Héma-Québec's Circular of Information. The Clinical Guide to Transfusion Chapter 20, Granulocyte transfusion therapy, also provides an overview of clinical evidence, indications and recent innovations in manufacturing of granulocyte concentrates.
General
1. When are granulocyte transfusions used?
Granulocyte transfusions may help eliminate infectious bacterial or fungal pathogens in some clinical settings. Granulocyte transfusions are primarily used as a supportive therapy in patients with significant and prolonged neutropenia (less than 0.5 x 109 /L) or documented neutrophil dysfunction and severe bacterial or fungal infection(s) that are not responding to antimicrobials.1
2. What is the evidence for the clinical efficacy of granulocyte transfusions?
The efficacy of granulocyte transfusions in various clinical settings has not been proven, either as a means to improve survival of patients or to shorten the duration of the infection. This product is not indicated for infection prophylaxis.1
3. Does Canadian Blood Services manufacture granulocyte concentrates?
No. Granulocyte concentrates are manufactured and distributed by Héma-Québec and transported directly to the ordering hospital. For hospitals outside the province of Québec, Canadian Blood Services will arrange the transport from Héma-Québec to the ordering hospital. Please refer to Héma-Québec's Circular of Information for additional information about granulocyte concentrates.
Ordering process
4. How can a hospital order granulocyte concentrates?
The ordering hospital must order granulocyte concentrates from Héma-Québec by completing two order forms provided on the Héma-Québec website. The forms must be retrieved from the Héma-Québec website as only the most recent forms will be accepted.
5. What forms do hospitals need to complete?
Complete both Héma-Québec forms ENR-03946 and ENR-03947:
- ENR-03946: Granulocytes apheresis request
- Under “Hospital Information,” leave “Customer no.” blank.
- “Requested quantity” refers to the number of granulocyte concentrates needed per day of treatment. Please indicate on this line if more than one granulocyte concentrate per day is required.
- Under “Additional Comments,” include a phone number or email address that can be used to communicate with the ordering hospital, as well as the fax number where Héma-Québec can send testing results.

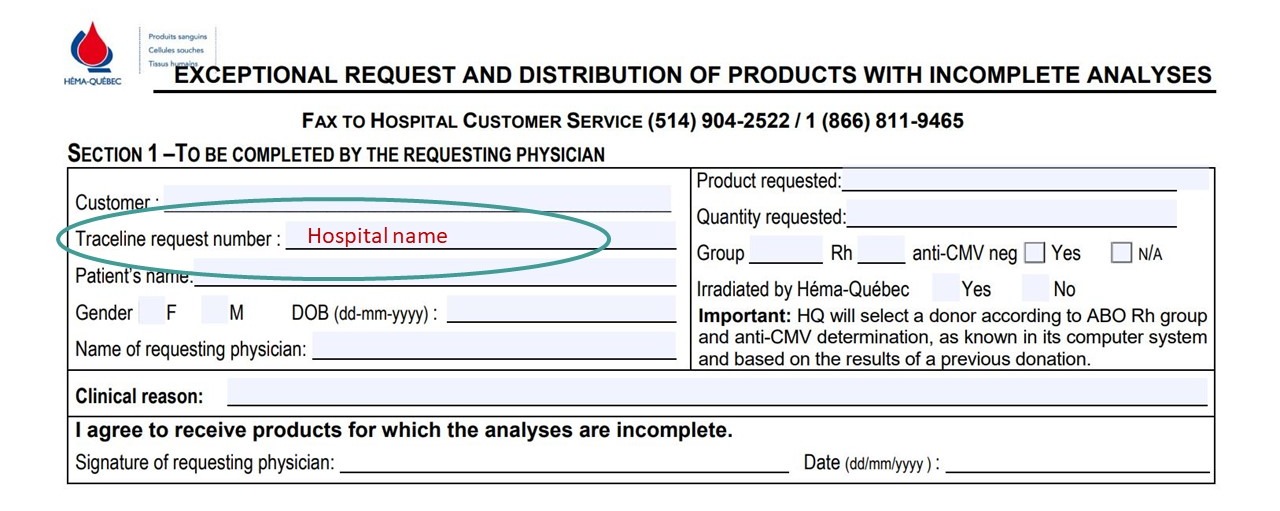
- ENR-03947: Exceptional request and distribution of products with incomplete analyses
- In section 1, under “Traceline request number,” enter hospital name.
- “Irradiated by Héma-Québec” can be used to indicate whether irradiation by Héma-Québec is required (circle Yes) or if the hospital is choosing to complete irradiation on-site (circle No).
- For CMV considerations, refer to the Héma-Québec Circular of Information.
6. Where should the hospital submit the granulocyte concentrate order forms?
Fax both forms (ENR-03946 and ENR-03947) to Héma-Québec at the number indicated on the bottom of form ENR-03946: 514-904-2522 or 1-866-811-9465.
7. After an order is submitted, what follow up is needed by the ordering hospital?
Two follow up actions must be taken by the ordering hospital:
- Inform Héma-Québec that a request for granulocyte concentrates has been submitted. This can be done by phone (514-832-5000 or 1-888-666-4362, ext. 6909) or email (sac.hopitaux@hema-quebec.qc.ca).
- Next, both completed order forms must be faxed to the local Canadian Blood Services distribution department.* This step is to inform Canadian Blood Services that a granulocyte concentrate order has been made.
*Note: Manitoba hospitals will fax both forms to the Canadian Blood Services Winnipeg Centre Diagnostic Services - Crossmatch Laboratory instead.
Collection, delivery and storage
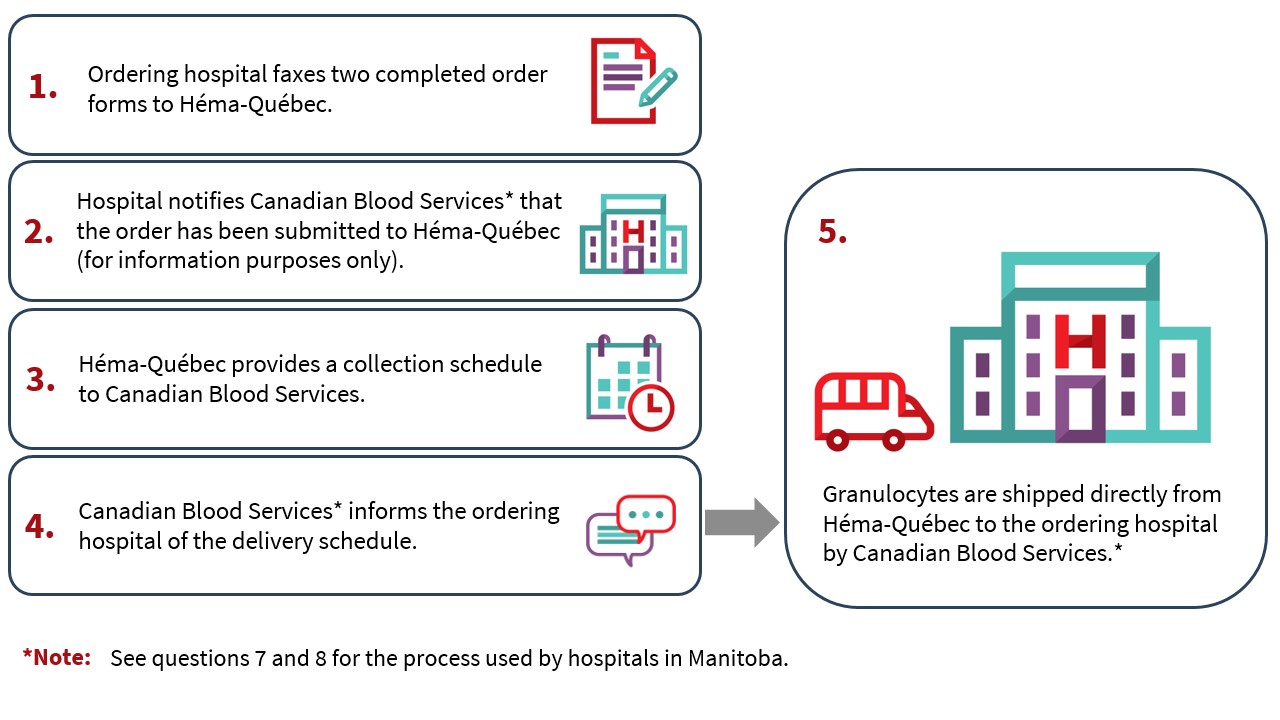
8. Who arranges delivery of granulocyte concentrates?
Canadian Blood Services will make arrangements with the ordering hospital for the delivery of granulocyte concentrates.*
*Note: For Manitoba hospitals, the Canadian Blood Services Winnipeg Diagnostic Services – Crossmatch Laboratory is responsible for issuing granulocyte concentrates and coordinating delivery.
9. Is the ordering hospital notified of collection and delivery schedules for granulocyte concentrates?
Héma-Québec and Canadian Blood Services will provide the ordering hospital with the granulocyte collection schedule as outlined in the process below:
- Héma-Québec will search for donors based on the granulocyte concentrate request.
- Granulocytes are generally collected in the morning and the manufactured granulocyte concentrate is shipped before end of day.
- Héma-Québec provides Canadian Blood Services with the date of granulocyte donation and pick-up from the collection site.
- After confirming the date for picking up the granulocyte concentrate from the Héma-Québec site, Canadian Blood Services* notifies the ordering hospital and Héma-Québec of the expected delivery date.
*Note: Manitoba hospitals receive product directly from the Canadian Blood Services Winnipeg Diagnostic Services – Crossmatch Laboratory, who will also coordinate the delivery.
10. How are granulocyte concentrates shipped?
Héma-Québec is responsible for packing the granulocyte concentrates. Canadian Blood Services is responsible for picking up the granulocyte concentrates from Héma-Québec and shipping to the ordering hospital at room temperature. For hospitals in the Ottawa region, Héma-Québec can arrange to have the boxes shipped back to Héma-Québec; hospitals located outside the Ottawa region can reach out to Héma-Québec to determine if the boxes should be discarded or returned to Héma-Québec.
A copy of the completed forms submitted to Héma-Québec (see question 4) will arrive with each granulocyte concentrate unit. Please refer to Héma-Québec's Circular of Information for additional information.

11. How are granulocytes stored?
Granulocyte concentrates are stored at 20°C to 24°C without agitation. Please refer to Héma-Québec's Circular of Information for additional information.
12. What is the shelf life of a granulocyte concentrate?
Granulocyte concentrates must be transfused within 24 hours of collection.
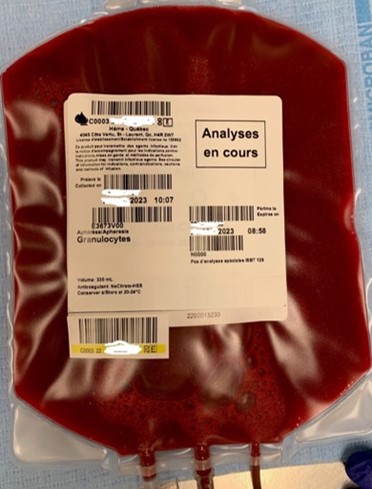
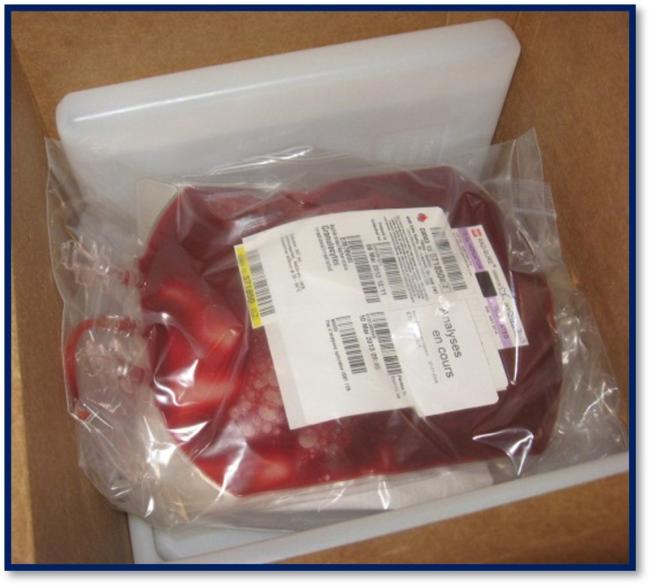
13. What information appears on the granulocyte label?
Granulocyte units are issued with transmissible disease testing results from the donor’s most recent blood donation (prior to granulocyte donation) and have “analyses en cours” (pending analysis) written on the ISBT label (refer to Figure 1). Blood group (ABO and Rh) information is not included on the granulocyte concentrate label.
Testing is performed on the donor’s granulocyte donation on the day following collection and the updated testing results are provided to the ordering hospital by fax as they become available. Hospitals should refer to this document for blood group and transmissible disease testing information.
The physician signature on form ENR-03947 indicates the hospital is willing to accept granulocyte concentrates before testing results are available.
Granulocyte concentrates are usually collected from frequent donors.
Testing results
14. Will the ordering hospital receive transmissible disease testing results?
Héma-Québec will forward donor testing results to the ordering hospital at the fax number provided by the hospital on order form ENR-03946. The ordering hospital is responsible for providing the fax number to ensure the results are received.
Testing is done on the weekday following the granulocytes collection. For example, granulocytes collected on Monday will have results available on Tuesday. If the granulocytes collection occurs on a Friday, Saturday, or public holiday, testing will be done on the next weekday.
Reporting requirements following granulocyte transfusion
15. What are the reporting requirements when granulocytes are requested?
For quality assurance (QA) purposes, data regarding patient diagnosis and outcome, the number of granulocyte concentrates transfused, and other relevant clinical details will be requested by Héma-Québec following the treatment.
For more information about the QA requirements, please contact the Héma-Québec medical office through hospital customer service at 514-832-5000 ext. 6909 and ask to speak to the on-call MD.
16. What if the patient has an adverse reaction to a granulocyte product?
Severe adverse reaction must be reported to Héma-Québec so they can conduct an investigation. The report must be emailed to riat@hema-quebec.qc.ca with an accompanying phone call (514-904-2522 or 514-904-8350 or 1-866-811-9465).
Suggested citation
Stepien J, Robert M-H. FAQ: Information for health professionals ordering granulocyte concentrates [Internet]. Ottawa: Canadian Blood Services; 2023 MM DD [cited YYYY MM DD]. Available from: FAQ: Information for health professionals ordering granulocyte concentrates | Professional Education (blood.ca)
References
- Héma-Québec. Circular of information. (Héma-Québec, 2021).
