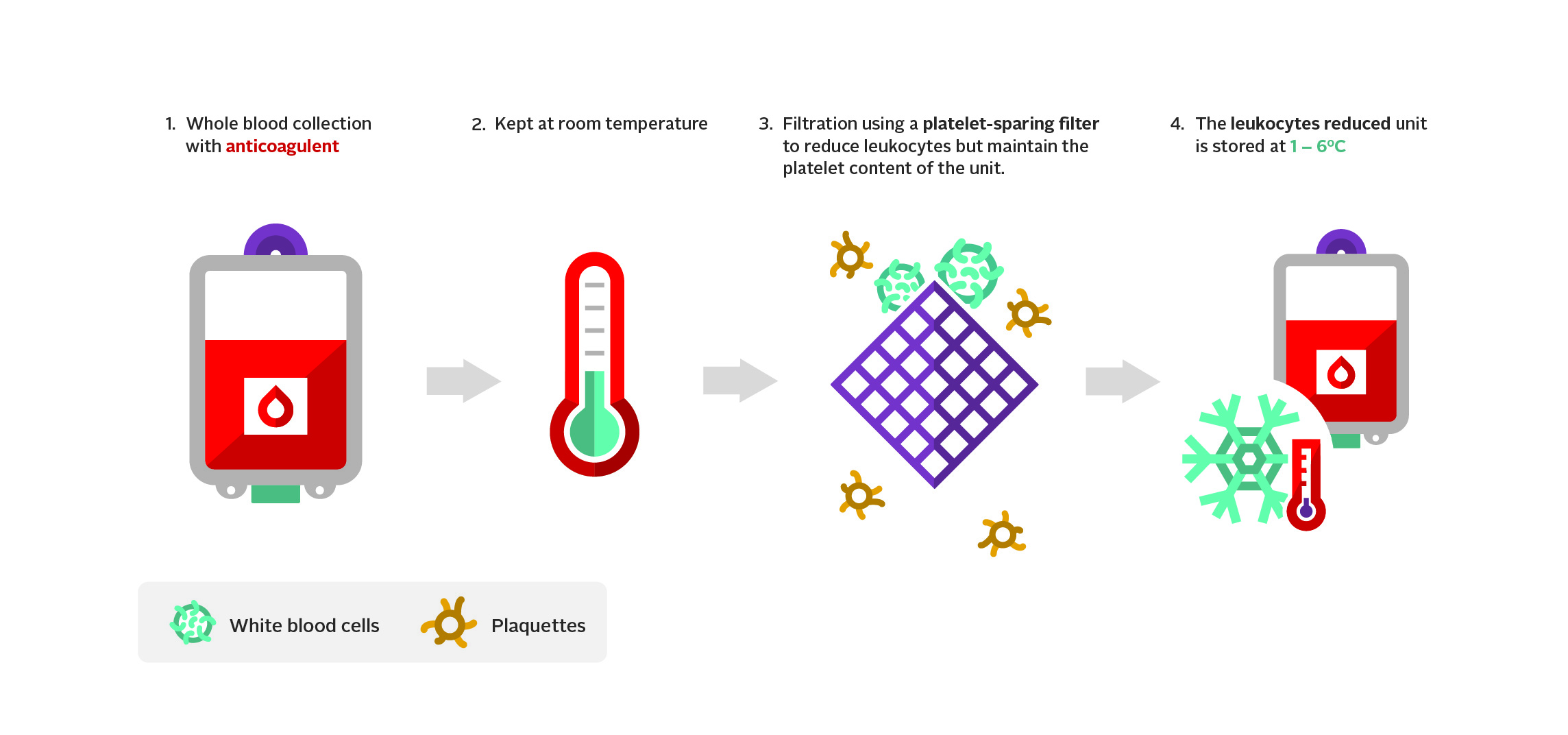
Table 1: Component characteristics of a typical unit of leukoreduced whole blood, red blood cells, pathogen-reduced, pooled platelets, pathogen-reduced apheresis platelets, and frozen plasma
| Component characteristic |
Leukoreduced whole blooda |
Red blood cellsb |
Pathogen-reduced pooled plateletsc |
Pathogen-reduced apheresis plateletsd |
Frozen plasmae |
S/D Plasma |
|---|
| Mean unit volume (mL) |
496 |
287 |
181 |
277 |
289 |
200 |
|---|
| Anticoagulant |
CPD* |
CPD* |
CPD* |
ACD-A* |
CPD* |
Sodium citrate |
|---|
| Approximate hematocrit (L/L) |
0.41 |
0.67 |
- |
- |
- |
- |
|---|
| Approximate hemoglobin (g) |
62 |
55 |
- |
- |
- |
- |
|---|
| Approximate platelet yield (x109 per unit) |
83 |
- |
243 |
252 |
- |
- |
|---|
| Approximate factor VIII (U/mL) |
0.78 |
- |
- |
- |
0.88 |
0 |
|---|
| Residual leukocytes (x106) |
0.2 |
0.06 |
0.04 |
<5 |
- |
- |
|---|
| Component of shelf life (from day of blood collection unless otherwise specified) |
21 days |
42 days |
7 days |
7 days |
12 months when frozen, 120 hours once thawed |
12 months when frozen, 120 hours once thawed |
|---|
* CPD: citrate phosphate dextrose; ACD-A: anticoagulant citrate dextrose, solution A
a known as Whole Blood, Leukocytes Reduced, in the Canadian Blood Services Circular of Information
b known as Red Blood Cells, Leukocytes Reduced (LR) in the Canadian Blood Services Circular of Information
c known as Pooled Platelets Psoralen Treated in the Canadian Blood Services Circular of Information
d known as Apheresis Platelets Psoralen Treated in the Canadian Blood Services Circular of Information
e known as Frozen Plasma CPD in the Canadian Blood Services Circular of Information