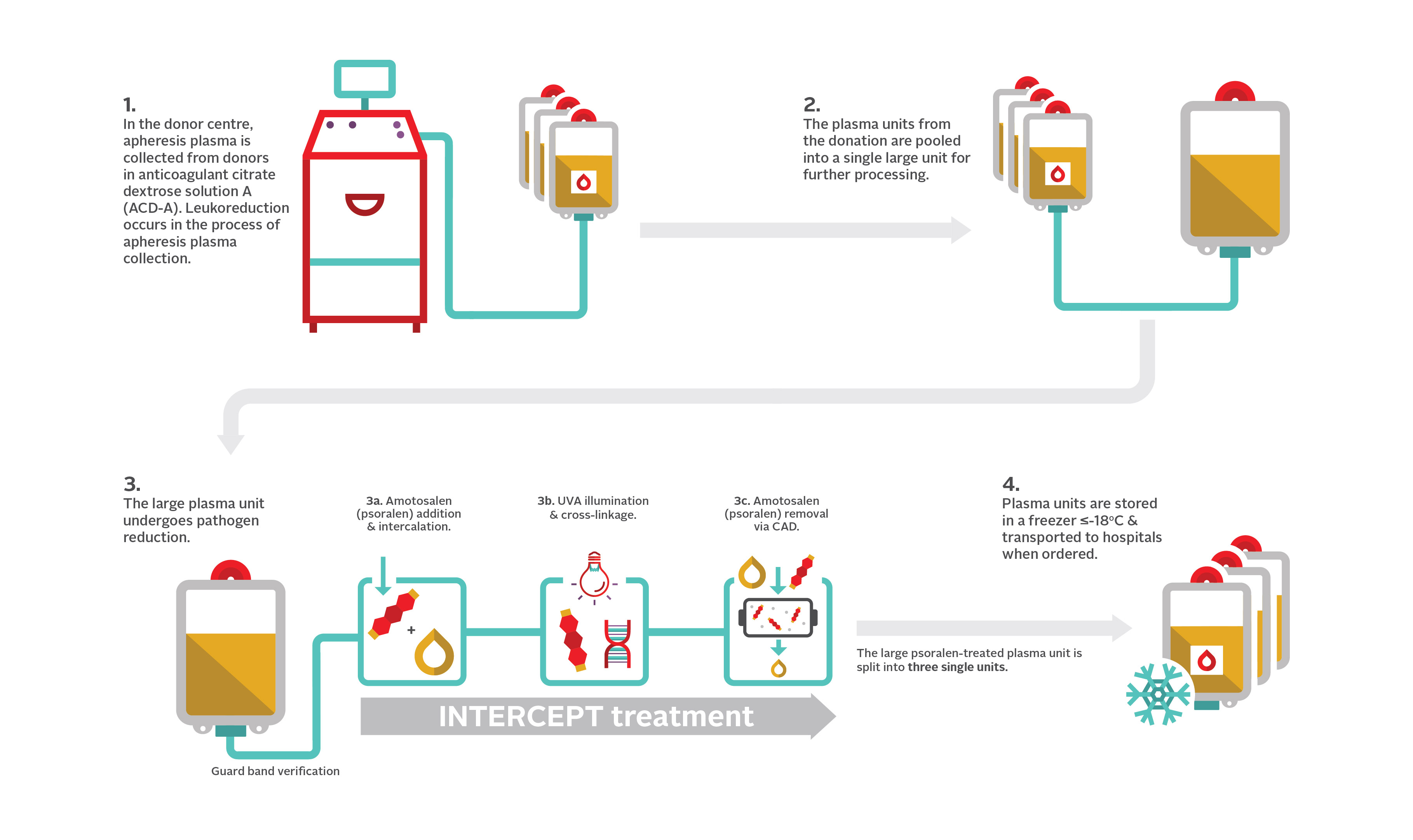
1. Cerus. (2016). INTERCEPT Blood system for plasma. https://intercept-canada.com/wp-content/uploads/sites/3/2021/02/INTERCEPT-CANADA-PACKAGE_INSERT-PLASMA_21may16.pdf
2. Terumo. (2017). Instructions for use 777019-935. In.
3. Prowse, C. V., de Korte, D., Hess, J. R., & van der Meer, P. F. (2014). Commercially available blood storage containers. Vox Sang, 106(1), 1-13. https://doi.org/10.1111/vox.12084
4. Walker, R. (2002). di(2-ethylhexyl) phthalate (DEHP) health Canada expert advisory panel on DEHP in medical devices. Canada.
5. Tomaselli, G. F., Mahaffey, K. W., Cuker, A., Dobesh, P. P., Doherty, J. U., Eikelboom, J. W., Florido, R., Gluckman, T. J., Hucker, W. J., Mehran, R., Messé, S. R., Perino, A. C., Rodriguez, F., Sarode, R., Siegal, D. M., & Wiggins, B. S. (2020). 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol, 76(5), 594-622. https://doi.org/10.1016/j.jacc.2020.04.053
6. Cuker, A., Burnett, A., Triller, D., Crowther, M., Ansell, J., Van Cott, E. M., Wirth, D., & Kaatz, S. (2019). Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol, 94(6), 697-709. https://doi.org/10.1002/ajh.25475
7. Green, L., Bolton-Maggs, P., Beattie, C., Cardigan, R., Kallis, Y., Stanworth, S. J., Thachil, J., & Zahra, S. (2018). British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol, 181(1), 54-67. https://doi.org/10.1111/bjh.15167
8. Association for the Advancement of Blood and Biotherapies (AABB). (2020). Pediatric Transfusion: A Physician's Handbook (Wong ECC, Ed. 5 ed.). Association for the Advancement of Blood and Biotherapies (AABB).
9. Sheffield, W. P., Bhakta, V., Mastronardi, C., Ramirez-Arcos, S., Howe, D., & Jenkins, C. (2012). Changes in coagulation factor activity and content of di(2-ethylhexyl)phthalate in frozen plasma units during refrigerated storage for up to five days after thawing. Transfusion, 52(3), 493-502. https://doi.org/10.1111/j.1537-2995.2011.03300.x
10. Scott, E., Puca, K., Heraly, J., Gottschall, J., & Friedman, K. (2009). Evaluation and comparison of coagulation factor activity in fresh-frozen plasma and 24-hour plasma at thaw and after 120 hours of 1 to 6°C storage. Transfusion, 49(8), 1584-1591. https://doi.org/10.1111/j.1537-2995.2009.02198.x
11. Sheffield, W. P., Bhakta, V., Yi, Q. L., & Jenkins, C. (2016). Stability of Thawed Apheresis Fresh-Frozen Plasma Stored for up to 120 Hours at 1°C to 6°C. J Blood Transfus, 2016, 6260792. https://doi.org/10.1155/2016/6260792
12. Bhakta, V., Jenkins, C., Ramirez-Arcos, S., & Sheffield, W. P. (2014). Stability of relevant plasma protein activities in cryosupernatant plasma units during refrigerated storage for up to 5 days postthaw. Transfusion, 54(2), 418-425. https://doi.org/10.1111/trf.12254
13. Serrano, K., Scammell, K., Weiss, S., Culibrk, B., Levin, E., Gyöngyössy-Issa, M., & Devine, D. V. (2010). Plasma and cryoprecipitate manufactured from whole blood held overnight at room temperature meet quality standards. Transfusion, 50(2), 344-353. https://doi.org/10.1111/j.1537-2995.2009.02441.x
14. Canadian Standards Association Group. (2025). CAN/CSA-Z902:25 Blood and blood components. CSA. https://www.csagroup.org/store/product/CAN-CSA-Z902%3A25/
15. Public Health Agency of Canada. Supplement guideline for investigation of suspected transfusion transmitted bacterial contamination. Canada Communicable Disease Report 2008; 34S1:1-8. http://www.phac-aspc.gc.ca/hcai-iamss/index-eng.php
16. Public Health Agency of Canada. (2025). Blood Safety Contribution Program. https://www.canada.ca/en/public-health/services/surveillance/blood-safety-contribution-program.html
17. Health Canada. (2025). Blood regulations (SOR/2013-178). Retrieved 2025-06-05, from https://laws-lois.justice.gc.ca/eng/regulations/sor-2013-178/index.html
18. Octapharma Canada. (2022). Octaplasma Product Monograph, October 31, 2022. https://www.octapharma.ca/en/therapies/product-overview
19. Erickson, A., Waldhaus, K., David, T., Huang, N., Rico, S., Corash, L., Mufti, N., & Benjamin, R. J. (2017). Plasma treated with amotosalen and ultraviolet A light retains activity for hemostasis after 5 days post-thaw storage at 1 to 6(o) C. Transfusion, 57(4), 997-1006. https://doi.org/10.1111/trf.13973
20. Heger, A., & Gruber, G. (2022). Frozen and freeze-dried solvent/detergent treated plasma: Two different pharmaceutical formulations with comparable quality. Transfusion, 62(12), 2621-2630. https://doi.org/10.1111/trf.17139
21. Mintz, P. D., Bass, N. M., Petz, L. D., Steadman, R., Streiff, M., McCullough, J., Burks, S., Wages, D., Van Doren, S., & Corash, L. (2006). Photochemically treated fresh frozen plasma for transfusion of patients with acquired coagulopathy of liver disease. Blood, 107(9), 3753-3760. https://doi.org/10.1182/blood-2004-03-0930
22. Mintz, P. D., Neff, A., MacKenzie, M., Goodnough, L. T., Hillyer, C., Kessler, C., McCrae, K., Menitove, J. E., Skikne, B. S., Damon, L., Lopez-Plaza, I., Rouault, C., Crookston, K. P., Benjamin, R. J., George, J., Lin, J. S., Corash, L., & Conlan, M. G. (2006). A randomized, controlled Phase III trial of therapeutic plasma exchange with fresh-frozen plasma (FFP) prepared with amotosalen and ultraviolet A light compared to untreated FFP in thrombotic thrombocytopenic purpura. Transfusion, 46(10), 1693-1704. https://doi.org/10.1111/j.1537-2995.2006.00959.x
23. de Alarcon, P., Benjamin, R., Dugdale, M., Kessler, C., Shopnick, R., Smith, P., Abshire, T., Hambleton, J., Matthew, P., Ortiz, I., Cohen, A., Konkle, B. A., Streiff, M., Lee, M., Wages, D., & Corash, L. (2005). Fresh frozen plasma prepared with amotosalen HCl (S-59) photochemical pathogen inactivation: transfusion of patients with congenital coagulation factor deficiencies. Transfusion, 45(8), 1362-1372. https://doi.org/10.1111/j.1537-2995.2005.00216.x
24. Cinqualbre, J., Kientz, D., Remy, E., Huang, N., Corash, L., & Cazenave, J. P. (2015). Comparative effectiveness of plasma prepared with amotosalen-UVA pathogen inactivation and conventional plasma for support of liver transplantation. Transfusion, 55(7), 1710-1720. https://doi.org/10.1111/trf.13100
25. Alhomsi, N., Shih, A., Zeller, M., St John, M., Webert, K., Yan, M., Ahmad, M., & Waito, M. (2025). Impact on solvent detergent plasma implementation on adverse transfusion reaction reporting. Canadian Society for Transfusion Medicine Annual Conference, St. John's, Newfoundland and Labrador.
26. Cazenave, J. P., Waller, C., Kientz, D., Mendel, I., Lin, L., Jacquet, M., Propst, M., Liu, W., Corash, L., Sundin, D., Defoin, L., Messe, N., & Osselaer, J. C. (2010). An active hemovigilance program characterizing the safety profile of 7483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment. Transfusion, 50(6), 1210-1219. https://doi.org/10.1111/j.1537-2995.2009.02579.x
27. Irsch, J., & Seghatchian, J. (2015). Update on pathogen inactivation treatment of plasma, with the INTERCEPT Blood System: Current position on methodological, clinical and regulatory aspects. Transfusion and Apheresis Science, 52(2), 240-244. https://doi.org/https://doi.org/10.1016/j.transci.2015.02.013
28. Ciaravino, V., Hanover, J., Lin, L., Sullivan, T., & Corash, L. (2009). Assessment of safety in neonates for transfusion of platelets and plasma prepared with amotosalen photochemical pathogen inactivation treatment by a 1-month intravenous toxicity study in neonatal rats. Transfusion, 49(5), 985-994. https://doi.org/10.1111/j.1537-2995.2008.02076.x
29. Corash, L., & Elliott, A. (2013). Routine transfusion experience in pediatric patients using platelet and plasma components treated for pathogrn inactivation: P-649. Vox Sang, 105, 282-283. https://www.ovid.com/journals/vxsan/abstract/00008111-201306001-00825~routine-transfusion-experience-in-pediatric-patients-using
30. Ciaravino, V., McCullough, T., Cimino, G., & Sullivan, T. (2003). Preclinical safety profile of plasma prepared using the INTERCEPT Blood System. Vox Sang, 85(3), 171-182. https://doi.org/https://doi.org/10.1046/j.1423-0410.2003.00351.x
31. McGonigle, A. M., Patel, E. U., Waters, K. M., Moliterno, A. R., Thoman, S. K., Vozniak, S. O., Ness, P. M., King, K. E., Tobian, A. A. R., & Lokhandwala, P. M. (2020). Solvent detergent treated pooled plasma and reduction of allergic transfusion reactions. Transfusion, 60(1), 54-61. https://doi.org/10.1111/trf.15600