Summary of adverse transfusion reactions 2020-2025
Author: Matthew Yan, MD, FRCPC
Revised: July 2025
According to Blood Regulations, hospitals are required to report adverse transfusion reactions following a transfusion to Canadian Blood Services if the reaction is thought to be caused by a blood component deficiency affecting its safety, and is attributable to a regulated activity at Canadian Blood Services (e.g., donor selection, blood collection, manufacturing). Hospitals also voluntarily report adverse reactions following transfusion through Transfusion Transmitted Injuries Surveillance System (TTISS) and/or as required to Health Canada, in cases where the reaction can be attributed to a regulated activity at the hospital that affected the safety of the blood component (e.g., pooling, washing, irradiation).
To learn more about adverse transfusion reactions, please visit Chapter 10 of the Clinical Guide to Transfusion.
How to report adverse transfusion reactions
To facilitate reporting of adverse transfusion reactions, we have prepared A Guide to Reporting Adverse Transfusion Reactions, which includes links to reporting forms.
Which adverse transfusion reactions are reported to Canadian Blood Services
The adverse transfusion reactions data reported to Canadian Blood Services are summarized in the graphs below. It is important to note that only adverse transfusion reactions that are reportable, or were initially deemed to be reportable, to Health Canada by Canadian Blood Services as determined by the Blood Regulations, are included in these graphs. Not included here are adverse reactions reported to Canadian Blood Services that are deemed to be not reportable to Health Canada (e.g., the reaction was minor or unrelated to the transfusion procedure).
Ongoing education with hospital health-care professionals promotes the appropriate reporting of transfusion reactions related to Canadian Blood Services’ components. Those efforts have led to a reduction in the number of adverse transfusion reactions reported to Canadian Blood Services that are related to the patient’s underlying condition (e.g., TACO or febrile reactions) rather than to the component.
Adverse reaction reports received by Canadian Blood Services may be revised as additional information becomes available. Therefore, the numbers reported for a given fiscal year may be subject to change.
The adverse transfusion reactions data shared by Canadian Blood Services are meant to supplement rather than replace the official reporting that comes from the TTISS program. Data are shown by fiscal year, which runs from April 1 until March 31 of the following year.
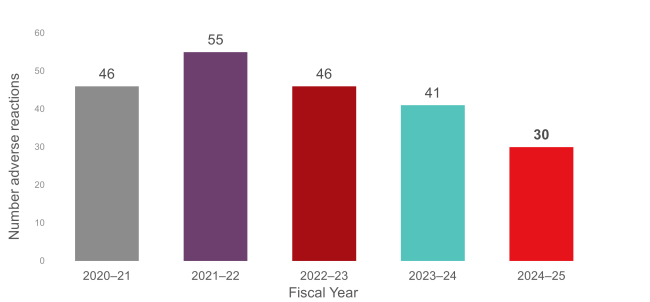
Figure 1: Total reportable† adverse reactions reported to Canadian Blood Services for the last five fiscal years.
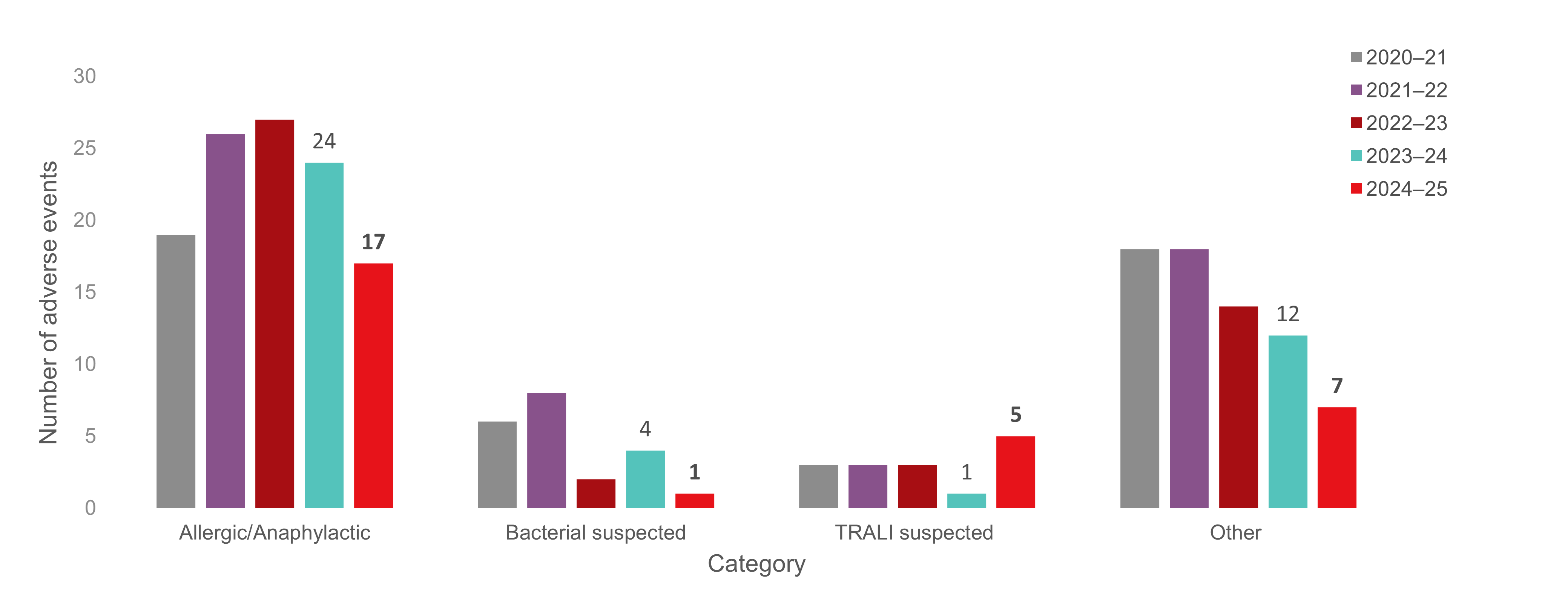
Figure 2: Reportable adverse reactions reported to Canadian Blood Services by category for the last five fiscal years.
Note: The "Other" category includes transfusion adverse reactions that were originally reported from Canadian Blood Services to Health Canada (namely suspected TRALIs), but following further investigation were reclassified as a reaction that is not reportable to Health Canada as per the Blood Regulations (due to underlying illness, TACO, etc. (TRALI=transfusion-related acute lung injury, TACO=transfusion associated circulatory overload).
Data on adverse transfusion reactions reported to Canadian Blood Services from 2020-21 to 2024-25, can be viewed in this downloadable Excel file:
Suggested citation
Yan M. Summary of adverse transfusion reactions 2020-2025 [Internet]. Ottawa: Canadian Blood Services; 2025 [cited YYYY MM DD]. Available from: Summary of adverse transfusion reactions 2020-2025 | Professional Education (blood.ca)