Chapter 3
Albumin
Background
Albumin is the most abundant protein in plasma and is synthesized in the liver at a rate of approximately 16g/day in a healthy adult. Several hormones can increase the body’s ability to synthesize albumin, but malnutrition, surgery, inflammation, medications and aging may all decrease production. Serum albumin is lost at a rate of 12g per 500 ml of blood lost; thus, in the setting of a four-unit hemorrhage (approximately half of the blood volume of an adult), the albumin lost will be entirely replaced by normal synthesis in three days.
Serum albumin is responsible for about 80% of the total plasma oncotic pressure (also known as colloid osmotic pressure). This pressure is important for maintaining appropriate levels of water in the circulatory system. Five percent albumin is isosmotic with plasma, but 25% albumin is hyperoncotic and is roughly equivalent to a plasma volume four- to five-fold higher than the infused volume. The intravascular volume expansion in a volume replete patient occurs within 20 minutes of the infusion of 25% albumin (i.e., 100 mL of 25% albumin theoretically expands quickly to 450 mL intravascular volume).
Product description
Intravenous albumin is prepared from donated human plasma using the Cohn cold ethanol fractionation process. Heat treatment is used for viral and other pathogen reduction. However, this may provide only partial protection from non-enveloped viruses (e.g., hepatitis A virus (HAV), parvovirus B19, etc.). Nucleic acid testing of plasma pools for parvovirus B19 and HAV has been implemented by most fractionators, with positive plasma units removed from the manufacturing process. See Chapter 7 of this Guide for more information on fractionated blood products.
When the glass container is intact, albumin is a sterile, latex-free solution with a physiologic pH and a known sodium concentration. Stabilizers are present however preservatives are not included. Normal albumin solutions are clear, slightly viscous fluids that range in color from almost colourless to pale yellow, amber or green (see Figure 1). See Table 1 for a list of albumin preparations available through Canadian Blood Services.
Figure 1. Albumin vials showcasing the range of colours in the appearance of albumin from almost colourless to pale yellow, amber or green. Special thanks to Dr. Kathryn Webert and Dr. Charles Musuka for sharing photographs of albumin vials which supported the development of this illustration.
Table 1. Albumin preparations available through Canadian Blood Services†
| Product name | Vial sizes | Supplier; Production location, plasma source | Storage | Stabilizers and buffers | Sodium (Na) content |
|---|---|---|---|---|---|
| Plasbumin 5% | 50 mL | Grifols; United States (US) for both manufacturing and plasma collection | 2 to 30°C | Sodium caprylate, acetyltryptophan | 130–160 mEq/L |
| Albumin 5% | 500 mL | ||||
| Albumin 25% | 100 mL | ||||
| Alburex 5% | 250 mL, 500 mL | CSL Behring; either manufactured in Switzerland or US from Canadian or US plasma donors | 2 to 30°C | Sodium caprylate, sodium N- acetyltryptophanate | 140 mEq/L |
| Alburex 5% | 50 mL, 100 mL |
† For ongoing updates, please refer to the complete table of plasma protein products at https://formulary.blood.ca/en
Efficacy of albumin
Albumin is used in a wide range of clinical scenarios and its use is highly variable between individual physicians and practice regions. In 2024, the International Collaboration for Transfusion Medicine Guidelines (ICTMG) published evidence-based clinical practice guidelines on the use of intravenous albumin.1 Twelve of the 14 recommendations in this guideline do not suggest albumin use in a wide variety of clinical situations where is it commonly transfused.
Critically ill patients
Among patients who are critically ill, research has found that intravenous albumin and alternatives result in similar outcomes for patients across a broad range of important patient variables (i.e., mortality, kidney failure, length of stay). As such, alternative fluids that are substantially cheaper and not derived from human plasma should be considered as first line therapy.
The Saline versus Albumin Fluid Evaluation (SAFE) trial, a large randomized controlled trial (RCT), enrolled 6,997 patients receiving fluid resuscitation with either 4% albumin or 0.9% normal saline in the intensive care unit (ICU). There was no significant difference in mortality between groups at 28 days (relative risk [RR] 0.99; 95% confidence interval [CI], 0.91–1.09) or significant differences in secondary outcomes including days spent in ICU, days spent in hospital, days of mechanical ventilation, and days of kidney replacement therapy (KRT).2 Subgroup analyses of the SAFE trial did not demonstrate any benefit of albumin in hypoalbuminemic patients. Lastly, albumin had a minimal fluid sparing effect over the first 4-days of measurement (ratio of saline: albumin was 1.38, rather than an expected ratio of 3:1).
Subsequent RCTs have produced similar results. In 2013, the Colloids Versus Crystalloids for the Resuscitation of the Critically Ill (CRISTAL) trial found no significant difference in 28 day mortality in ICU patients with hypovolemia who were treated with colloids (including albumin) versus crystalloids (RR, 0.96; 95% CI, 0.88–1.04).3 In the Albumin Italian Outcome Sepsis (ALBIOS) study that enrolled 1,818 patients with severe sepsis or septic shock to receive either 20% albumin in addition to crystalloid therapy or crystalloid solution alone, there was no improvement in rate of survival at 28 days (RR 1.0; 95% CI 0.85–1.05).4 Lastly, a systematic review from 2019, which included 55 RCTs and 26,329 patients, compared crystalloids with colloids in critical care and found no mortality improvement when comparing crystalloids to albumin (RR 1.02; 95% CI, 0.96–1.10).5
Patients undergoing kidney replacement therapy
Intravenous albumin is not suggested for the prevention or treatment of intradialytic hypotension (IDH) or for improving ultrafiltration.1 A Cochrane review of one RCT comparing albumin to normal saline in 45 patients with a previous history of IDH found no difference in treatment of symptomatic hypotension during dialysis or percentage target ultrafiltration achieved.6 A 2021 crossover trial of 65 hospitalized patients requiring hemodialysis with albumin levels <30g/L found that hypotension, lowest intradialytic systolic blood pressure, and ultrafiltration were improved with 25% albumin compared to saline. However, as it was a crossover trial, patient outcomes could not be assessed.7 In a 2021 blinded, parallel-group trial critically ill patients were randomized to 25% albumin versus 0.9% saline administered as 100 ml boluses twice during 8 hour slow, low-efficiency dialysis (SLED) treatments.8 The trial included 60 patients with acute kidney injury (AKI) who underwent 271 SLED treatments in the ICU. Those randomized to albumin had less hypotension during and after SLED treatments. No significant differences were observed in the achieved ultrafiltration volumes.
Given the costs of albumin, frequent need for treatment (i.e., 3x per week) for maintenance hemodialysis, and an overall lack of evidence to support superiority, alternative fluids or treatments (i.e., midodrine, high dialysate calcium, lower dialysate temperature, individualized ultrafiltration rates) should be considered. There is an ongoing RCT comparing 20–25% albumin to normal saline in critically ill patients with AKI requiring KRT to determine if albumin improves IDH and ultrafiltration (ALTER-AKI, NCT 04705896).9
Cirrhosis
Large volume paracentesis
In patients with cirrhosis and ascites, the use of albumin for large-volume paracentesis (>5L) is recommended to prevent paracentesis-induced circulatory dysfunction (defined as an increase in the plasma renin activity >50% of the pre-treatment value to a level >4ng/mL/h on the sixth day after paracentesis).1, 10, 11 A 2019 Cochrane systematic review looking at the use of any plasma expanders in patients with cirrhosis undergoing large volume paracentesis showed reduced incidence of post-paracentesis circulatory dysfunction, however there was no effect on mortality, kidney impairment, or recurrence of ascites.12
Spontaneous bacterial peritonitis (SBP)
In patients with cirrhosis and SBP, albumin is recommended to reduce kidney impairment and mortality. Two systematic reviews identified five RCTs in patients with cirrhosis and SBP receiving variable doses of hyperoncotic albumin, where albumin reduced the rate of kidney impairment and mortality.13, 14 However, these RCTs were of small sample sizes and the control arm did not have explicit fluid resuscitation.1
Extraperitoneal infections
Albumin is not recommended in patients with cirrhosis and extraperitoneal infections. Two systematic reviews of RCTs comparing albumin plus antibiotics to antibiotics alone found no improvement in mortality or kidney impairment, and increased risk of pulmonary edema. 15, 16
Hepatorenal syndrome (HRS) Type 1
Two systematic reviews on the treatment of HRS did not find any RCTs comparing albumin to placebo or no treatment.17, 18 Instead, all studies have included albumin in both treatment and control arms, while evaluating a variety of drug therapies that included terlipressin, midodrine, and octreotide. This does not allow for an evaluation of albumin and its effect on outcomes in HRS, however, it is commonly prescribed. No recommendations regarding the use of albumin for patients with cirrhosis and HRS could be made in the most recent ICTMG albumin guidelines. 1
Long-term albumin administration
The ICTMG recommends against the routine use of albumin in outpatients with cirrhosis and uncomplicated ascites despite diuretic therapy.1 The ANSWER (Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis) trial randomized patients with cirrhosis and uncomplicated ascites who were treated with anti-aldosteronic drugs and furosemide, to receive standard medical treatment (SMT) or SMT plus albumin (40g twice weekly for 2 weeks, then 40g weekly).20 They found increased overall survival in the albumin group (77% vs. 66% survival at 18 months, HR 0.62, 95% CI 0.40-0.95). A criticism of this study is that the albumin treated patients underwent weekly health assessments, and the control group did not. Another trial in patients with decompensated cirrhosis waiting for liver transplant randomized patients to midodrine and albumin (40g every 15 days) or placebo and found no significant differences between both groups in terms of mortality or probability of developing complications of cirrhosis.19 In this study there was the same health case exposure in both groups. An additional RCT (PRECIOSA) is underway in this population.1, 21
Cardiac surgery
Intravenous albumin is not recommended in patients undergoing cardiac surgery for priming the cardiopulmonary bypass (CPB) circuit or volume replacement due to lack of efficacy and increased harm.1 A systematic review and meta-analysis of RCTs in pediatric and adult patients undergoing cardiovascular surgery comparing albumin with gelatin, starches, or crystalloid solutions for priming the CPB circuit and/or volume expansion, did not show improvement in mortality, kidney failure, blood loss, ICU length of stay, hospital length of stay, or blood component use.22 Subsequent to this systematic review, two large RCTs were published. The first confirmed that adding 4% albumin to the CPB priming solution did not decrease the incidence of cardiac surgery associated AKI.23 The second RCT found that 20% albumin did not significantly reduce the duration of vasopressor therapy compared to crystalloid.24 The largest trial in this population, Albumin in Cardiac Surgery (ALBICS), compared 4% albumin to Ringer acetate for priming of the CPB circuit and perioperative intravenous volume replacement. This study found significantly higher rates of bleeding, resternotomy, and infection in the albumin group.25
Recent evidence, published after the ICTMG albumin guidelines, do not support the routine use of postoperative hypertonic albumin in patients undergoing high-risk cardiac surgery. 26 The ALBICS AKI trial evaluated the effect of postoperative 20% albumin compared with usual care on the occurrence of AKI in high-risk cardiac surgery patients. They found that AKI occurred in more patients in the albumin than the usual care group (48.9% vs. 43.4%, strata-adjusted relative risk 1.12, 95% CI 1.04-1.21, p=0.003).26 In addition, there was also more blood transfusion in the albumin group (37.8% vs. 29.9%, p=0.04) but no other differences in secondary outcomes.26
Safety of albumin
There are specific patient populations where albumin has been shown to not improve outcomes and instead cause patient harm, detailed in Table 2. A rare side effect of albumin is anaphylaxis.27 There have been no reports of human immunodeficiency virus (HIV), hepatitis or other viral transmission at the time of writing, but a small theoretical risk of variant Creutzfeldt-Jakob disease (vCJD) transmission exists.28
Table 2. Harms of intravenous albumin29
| Patient population | Comparison | Risk(s) in albumin group |
|---|---|---|
| Cardiac surgery | ||
| On-pump cardiac surgery25 | 4% albumin vs. Ringer acetate | Increased risk of bleeding, resternotomy, and infection |
| High-risk cardiac surgery, postoperative 26 | 20% albumin vs. Usual care | Increased risk of acute kidney injury, blood transfusion |
| Cirrhosis | ||
| Decompensated cirrhosis (inpatient) and albumin <30g/L30 | 20% albumin vs. Standard of care | Increased serious or life-threatening serious adverse events (pulmonary edema, fluid overload) |
| Cirrhosis and extraperitoneal infections16 | 20% albumin vs. Control | Increased pulmonary edema |
| Critically ill patients | ||
| Traumatic brain injury29 | 4% albumin vs. Normal saline | Increased mortality |
Indications
See Table 3 and 4 for recommended indications for use of 25% and 5% intravenous albumin, respectively.
Table 3. Indications for 25% albumin preparations.
| Indication | Benefits |
|---|---|
| Liver cirrhosis | |
| Large volume (>5L) paracentesis | Prevention of paracentesis induced circulatory dysfunction |
| Spontaneous bacterial peritonitis | Reduced rate of kidney impairment and mortality |
| Hepatorenal syndrome type 1 | No data available comparing albumin vs. alternative strategy |
Table 4. Indications for 5% albumin preparations
| Indication | Benefits |
|---|---|
| Therapeutic apheresis 31 | Replacement fluid for plasma exchange (excluding patients with thrombotic thrombocytopenic purpura, coagulopathy, or bleeding) |
| Thermal injury >20% total body surface area† | May reduce total volume of resuscitation fluids and improve urine output when used in the first 24 hours for resuscitation† |
† American Burn Association Clinical practice guidelines recommend clinicians consider use of albumin, especially in patients with larger burns.
There is no strong evidence to support the use of albumin in the following circumstances:
• cardiac surgery (priming CPB circuit or volume replacement, and postoperative 20% albumin)
• first line volume resuscitation for hypovolemia
• cerebral ischemia
• hypoalbuminemia
• in conjunction with diuretics for removal of extravascular fluid
• treatment or prevention of intradialytic hypotension or ultrafiltration
• patients with cirrhosis and extra-peritoneal infections
• traumatic brain injury
Contraindications
Albumin is contraindicated in:
• Patients who would not tolerate a rapid increase in circulating blood volume.
• Patients with a history of a serious allergic reaction to albumin.
Dose and administration
The volume and rate of infusion should be determined by the clinical situation. However, the infusion rates for 5% albumin solutions should not exceed 5ml per minute; whereas the rate for 25% albumin should not exceed 1–2ml per minute, due to its hyperosmotic nature. Monitoring patients for pulmonary edema and circulatory overload is recommended with 25% albumin infusions. Suggested albumin doses are indicated in Table 5.
Table 5. Suggested maximum doses† for indicated uses of 25% albumin32.
| Indication | Dose |
|---|---|
| Liver cirrhosis | |
| Large volume (>5L) paracentesis | 6–8 g per liter of fluid removed |
| Spontaneous bacterial peritonitis | Day 1: 1.5 g/kg Day 3: 1 g/kg |
| Hepatorenal syndrome type 1‡ | Day 1: 1 g/kg Days 2–14: 25–50g (100–200mL) per day |
†Consider adjusting the dose in patients at risk of fluid overload
‡Administered with vasoactive agents (e.g. midodrine or terlipressin)
Infusion is through a standard vented intravenous (IV) set. Albumin is compatible with standard electrolyte and carbohydrate IV solutions such as normal saline, Ringer’s lactate, PlasmaLyte and D5W, but should not be co-infused with solutions containing alcohol or protein hydrolysates. Albumin must not be diluted with hypotonic solutions such as sterile water for injection, as it may lead to severe hemolysis. Once opened, the vial of albumin should be discarded if not infused within four hours.
Storage and transportation
See Table 1 for storage temperatures for the various albumin preparations available through Canadian Blood Services. The shelf-life ranges from two to five years depending on the manufacturing process. An expiry date is stated on each package and the expiration date of each vial should be checked prior to administration. The product must be transported while maintaining the temperature within the range specified in the package insert for the particular brand and to prevent breakage. Figure 2 lists cases when the albumin vial should not be administered.
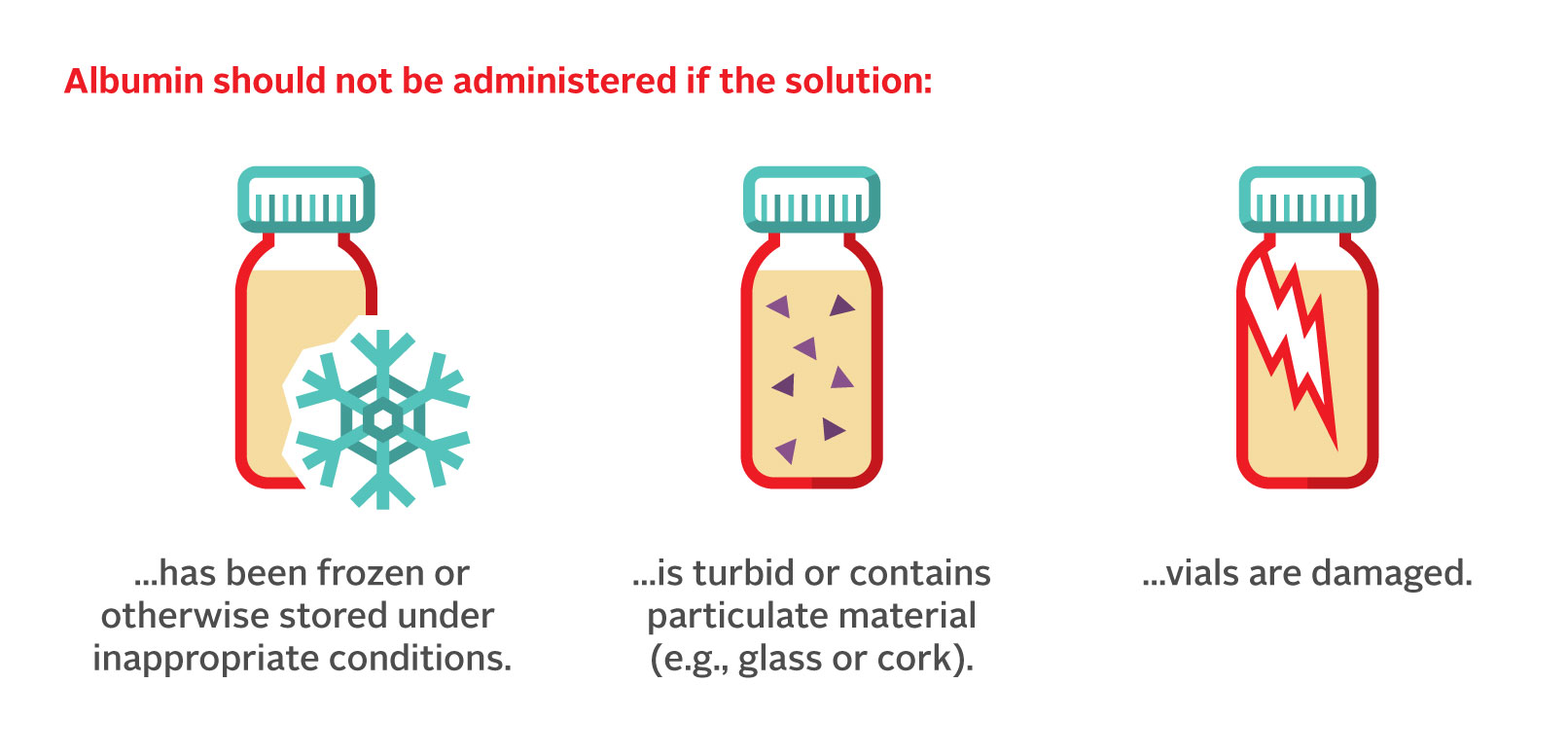
Figure 2. Cases where the albumin vial has been compromised and should not be administered for treatment.
Alternatives to albumin
Alternatives to albumin therapy include:
• crystalloid (e.g., normal saline, Ringer’s lactate)
• colloid (e.g., hydroxyethyl starches)
The advantages of crystalloid therapy over most colloid solutions include decreased cost and increased accessibility. The disadvantages of crystalloids are primarily seen in situations requiring large volumes for resuscitation, which may lead to peripheral and pulmonary edema.
Although other colloids such as hydroxyethyl starches (HES) are cheaper than albumin, they may be associated with increased side effects.33 In 2013, a Health Canada advisory was issued advising clinicians that increased mortality, renal injury, and liver failure have been associated with the use of HES solutions and that HES solutions are now contraindicated in patients with sepsis, severe liver disease or renal impairment with oliguria and anuria, not related to hypovolemia.34
Additional resources
- ICTMG guideline on the use of intravenous albumin: Callum et al. Use of intravenous albumin: A guideline from the international collaboration for transfusion medicine guidelines. doi: 10.1016/j.chest.2024.02.049 https://journal.chestnet.org/article/S0012-3692(24)00285-X/fulltext
- For additional implementation materials, please see the ICTMG website, https://www.ictmg.org/albumin
- Please also see the article, Use of albumin in critically ill patients published in Chest Physician, Critical Care https://www.chestphysician.org/use-of-albumin-in-critically-ill-patients/
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Sciences’ Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Acknowledgements
The authors Nicole Relke and Jeannie Callum, acknowledge Gwen Clarke, MD, FRCPC and Matthew Yan, MD, FRCPC as authors of a previous version of this chapter.
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Clinical Guide feedback form.
Suggested citation
Relke, N ., & Callum, M. (2025). Albumin. In A. Khandelwal & K. Brooks (Eds.), Clinical guide to transfusion (Chap. 3). Canadian Blood Services. https://professionaleducation.blood.ca/en/transfusion/clinical-guide/albumin
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
References
1. Callum, J., Skubas, N. J., Bathla, A., Keshavarz, H., Clark, E. G., Rochwerg, B., Fergusson, D., Arbous, S., Bauer, S. R., China, L., Fung, M., Jug, R., Neill, M., Paine, C., Pavenski, K., Shah, P. S., Robinson, S., Shan, H., Szczepiorkowski, Z. M., . . . Shehata, N. (2024). Use of Intravenous Albumin: A Guideline From the International Collaboration for Transfusion Medicine Guidelines. Chest, 166(2), 321-338. https://doi.org/10.1016/j.chest.2024.02.049
2. Finfer, S., Bellomo, R., Boyce, N., French, J., Myburgh, J., & Norton, R. (2004). A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med, 350(22), 2247-2256. https://doi.org/10.1056/NEJMoa040232
3. Annane, D., Siami, S., Jaber, S., Martin, C., Elatrous, S., Descorps Declère, A., Preiser, J. C., Outin, H., Troché, G., Charpentier, C., Trouillet, J. L., Kimmoun, A., Forceville, X., Darmon, M., Lesur, O., Reignier, J., Abroug, F., Berger, P., Clec’h, C., . . . for the CRISTAL Investigators. (2013). Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: The cristal randomized trial. JAMA, 310(17), 1809-1817. https://doi.org/10.1001/jama.2013.280502
4. Caironi, P., Tognoni, G., Masson, S., Fumagalli, R., Pesenti, A., Romero, M., Fanizza, C., Caspani, L., Faenza, S., Grasselli, G., Iapichino, G., Antonelli, M., Parrini, V., Fiore, G., Latini, R., & Gattinoni, L. (2014). Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med, 370(15), 1412-1421. https://doi.org/10.1056/NEJMoa1305727
5. Martin, G. S., & Bassett, P. (2019). Crystalloids vs. colloids for fluid resuscitation in the Intensive Care Unit: A systematic review and meta-analysis. J Crit Care, 50, 144-154. https://doi.org/10.1016/j.jcrc.2018.11.031
6. Fortin, P. M., Bassett, K., & Musini, V. M. (2010). Human albumin for intradialytic hypotension in haemodialysis patients. Cochrane Database Syst Rev(11), Cd006758. https://doi.org/10.1002/14651858.CD006758.pub2
7. Macedo, E., Karl, B., Lee, E., & Mehta, R. L. (2021). A randomized trial of albumin infusion to prevent intradialytic hypotension in hospitalized hypoalbuminemic patients. Crit Care, 25(1), 18. https://doi.org/10.1186/s13054-020-03441-0
8. Clark, E. G., McIntyre, L., Watpool, I., Kong, J. W. Y., Ramsay, T., Sabri, E., Canney, M., Hundemer, G. L., Brown, P. A., Sood, M. M., & Hiremath, S. (2021). Intravenous albumin for the prevention of hemodynamic instability during sustained low-efficiency dialysis: a randomized controlled feasibility trial (The SAFER-SLED Study). Ann Intensive Care, 11(1), 174. https://doi.org/10.1186/s13613-021-00962-x
9. Clark, E. G. (2025). Albumin To Enhance Recovery After Acute Kidney Injury (ALTER-AKI), NCT04705896. National Library of Medicine (NLM). Retrieved 2025-01-12 from https://clinicaltrials.gov/study/NCT04705896?term=ALTER-AKI&intr=albumin&rank=2&a=4
10. Biggins, S. W., Angeli, P., Garcia-Tsao, G., Ginès, P., Ling, S. C., Nadim, M. K., Wong, F., & Kim, W. R. (2021). Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology, 74(2), 1014-1048. https://doi.org/10.1002/hep.31884
11. European Association for the Study of the Liver (EASL). (2018). EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol, 69(2), 406-460. https://doi.org/10.1016/j.jhep.2018.03.024
12. Simonetti, R. G., Perricone, G., Nikolova, D., Bjelakovic, G., & Gluud, C. (2019). Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis. Cochrane Database Syst Rev, 6(6), Cd004039. https://doi.org/10.1002/14651858.CD004039.pub2
13. Zaccherini, G., Tufoni, M., & Bernardi, M. (2020). Albumin Administration is Efficacious in the Management of Patients with Cirrhosis: A Systematic Review of the Literature. Hepat Med, 12, 153-172. https://doi.org/10.2147/hmer.S264231
14. Salerno, F., Navickis, R. J., & Wilkes, M. M. (2013). Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol, 11(2), 123-130.e121. https://doi.org/10.1016/j.cgh.2012.11.007
15. Leão, G. S., John Neto, G., Jotz, R. F., Mattos, A. A., & Mattos Â, Z. (2019). Albumin for cirrhotic patients with extraperitoneal infections: A meta-analysis. J Gastroenterol Hepatol, 34(12), 2071-2076. https://doi.org/10.1111/jgh.14791
16. Wong, Y. J., Qiu, T. Y., Tam, Y. C., Mohan, B. P., Gallegos-Orozco, J. F., & Adler, D. G. (2020). Efficacy and Safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: A systematic review and meta-analysis. Dig Liver Dis, 52(10), 1137-1142. https://doi.org/10.1016/j.dld.2020.05.047
17. Best, L. M., Freeman, S. C., Sutton, A. J., Cooper, N. J., Tng, E. L., Csenar, M., Hawkins, N., Pavlov, C. S., Davidson, B. R., Thorburn, D., Cowlin, M., Milne, E. J., Tsochatzis, E., & Gurusamy, K. S. (2019). Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev, 9(9), Cd013103. https://doi.org/10.1002/14651858.CD013103.pub2
18. Thomson, M. J., Taylor, A., Sharma, P., Lok, A. S., & Tapper, E. B. (2020). Limited Progress in Hepatorenal Syndrome (HRS) Reversal and Survival 2002-2018: A Systematic Review and Meta-Analysis. Dig Dis Sci, 65(5), 1539-1548. https://doi.org/10.1007/s10620-019-05858-2
19. Solà, E., Solé, C., Simón-Talero, M., Martín-Llahí, M., Castellote, J., Garcia-Martínez, R., Moreira, R., Torrens, M., Márquez, F., Fabrellas, N., de Prada, G., Huelin, P., Lopez Benaiges, E., Ventura, M., Manríquez, M., Nazar, A., Ariza, X., Suñé, P., Graupera, I., . . . Ginès, P. (2018). Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol, 69(6), 1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
20. Caraceni, P., Riggio, O., Angeli, P., Alessandria, C., Neri, S., Foschi, F. G., Levantesi, F., Airoldi, A., Boccia, S., Svegliati-Baroni, G., Fagiuoli, S., Romanelli, R. G., Cozzolongo, R., Di Marco, V., Sangiovanni, V., Morisco, F., Toniutto, P., Tortora, A., De Marco, R., . . . Bernardi, M. (2018). Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet, 391(10138), 2417-2429. https://doi.org/10.1016/s0140-6736(18)30840-7
21. LLC, G. T. (2025). Effects of Long-Term Administration of Human Albumin in Participants With Decompensated Cirrhosis and Ascites (PRECIOSA).
22. Skubas, N. J., Callum, J., Bathla, A., Keshavarz, H., Fergusson, D., Wu, B., Stanworth, S., & Shehata, N. (2024). Intravenous albumin in cardiac and vascular surgery: a systematic review and meta-analysis. Br J Anaesth, 132(2), 237-250. https://doi.org/10.1016/j.bja.2023.11.009
23. Miralles Bagán, J., Parrilla Quiles, L., Paniagua Iglesias, P., Betbesé Roig, A. J., Sabaté Tenas, S., Pérez García, S., & García Álvarez, M. (2025). The Potential Role of Albumin in Reducing Cardiac Surgery–Associated Acute Kidney Injury: A Randomized Controlled Trial. J Cardiothorac Vasc Anesth, 39(2), 453-460. https://doi.org/10.1053/j.jvca.2024.10.012
24. Wigmore, G. J., Deane, A. M., Presneill, J. J., Eastwood, G., Serpa Neto, A., Maiden, M. J., Bihari, S., Baker, R. A., Bennetts, J. S., Ghanpur, R., Anstey, J. R., Raman, J., & Bellomo, R. (2024). Twenty percent human albumin solution fluid bolus administration therapy in patients after cardiac surgery-II: a multicentre randomised controlled trial. Intensive Care Med, 50(7), 1075-1085. https://doi.org/10.1007/s00134-024-07488-3
25. Pesonen, E., Vlasov, H., Suojaranta, R., Hiippala, S., Schramko, A., Wilkman, E., Eränen, T., Arvonen, K., Mazanikov, M., Salminen, U.-S., Meinberg, M., Vähäsilta, T., Petäjä, L., Raivio, P., Juvonen, T., & Pettilä, V. (2022). Effect of 4% Albumin Solution vs Ringer Acetate on Major Adverse Events in Patients Undergoing Cardiac Surgery With Cardiopulmonary Bypass: A Randomized Clinical Trial. JAMA, 328(3), 251-258. https://doi.org/10.1001/jama.2022.10461
26. Shehabi, Y., Balachandran, M., Al-Bassam, W., Bailey, M., Bellomo, R., Bihari, S., Brown, A., Brown, A., Collins, D., Darlison, P. R., Li, M. A., Mandarano, R., Sarode, V., & Pakavakis, A. (2025). Postoperative 20% Albumin Infusion and Acute Kidney Injury in High-Risk Cardiac Surgery Patients: The ALBICS AKI Randomized Clinical Trial. JAMA Surg. https://doi.org/10.1001/jamasurg.2025.1683
27. Shimode, N., Yasuoka, H., Kinoshita, M., Hirashima, K., Tsujimoto, S., Tashiro, C., & Kokubunji, A. (2006). Severe anaphylaxis after albumin infusion in a patient with ahaptoglobinemia. Anesthesiology, 105(2), 425-426. https://doi.org/10.1097/00000542-200608000-00027
28. U.S. Food & Drug Administration. (2018). Potential Risk of Variant Creutzfeldt-Jakob Disease (vCJD) From Plasma-Derived Products. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/potential-risk-variant-creutzfeldt-jakob-disease-vcjd-plasma-derived-products
29. Safe Study Investigators, Australian New Zealand Intensive Care Society Clinical Trials Group, Australian Red Cross Blood Service, George Institute for International Health, Myburgh, J., Cooper, D. J., Finfer, S., Bellomo, R., Norton, R., Bishop, N., Kai Lo, S., & Vallance, S. (2007). Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med, 357(9), 874-884. https://doi.org/10.1056/NEJMoa067514
30. China, L., Freemantle, N., Forrest, E., Kallis, Y., Ryder, S. D., Wright, G., Portal, A. J., Becares Salles, N., Gilroy, D. W., & O'Brien, A. (2021). A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N Engl J Med, 384(9), 808-817. https://doi.org/10.1056/NEJMoa2022166
31. Oliver, M., & Patriquin, C. (2022). Chapter 14 Therapeutic apheresis (Khandelwal and Abe, Eds.). Canadian Blood Services. https://professionaleducation.blood.ca/en/transfusion/clinical-guide/therapeutic-apheresis
32. Sort, P., Navasa, M., Arroyo, V., Aldeguer, X., Planas, R., Ruiz-del-Arbol, L., Castells, L., Vargas, V., Soriano, G., Guevara, M., Gines, P., & Rodes, J. (1999). Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med, 341(6), 403-409. https://doi.org/10.1056/nejm199908053410603
33. Reine, P. A., Kongsgaard, U. E., Andersen, A., Thogersen, A. K., & Olsen, H. (2010). Infusions of albumin increase free fraction of naproxen in healthy volunteers: a randomized crossover study. Acta Anaesthesiol Scand, 54(4), 430-434. https://doi.org/10.1111/j.1399-6576.2009.02142.x
34. Stange, J., Stiffel, M., Goetze, A., Strube, S., Gruenert, J., Klammt, S., Mitzner, S., Koball, S., Liebe, S., & Reisinger, E. (2011). Industrial stabilizers caprylate and N-acetyltryptophanate reduce the efficacy of albumin in liver patients. Liver Transpl, 17(6), 705-709. https://doi.org/10.1002/lt.22237