Anaphylactic transfusion reactions and IgA deficiency
Authors: Aditi Khandelwal, MDCM, FRCPC; Gwen Clarke, MD, FRCPC; Mindy Goldman, MD, FRCPC
Key Points
- Severe allergic or anaphylactic reactions are reported in 1.2 to 5.9 per 100,000 components transfused. The most recent Canadian Blood Services surveillance data on allergic reactions is available in the annual surveillance report.
- There are various mechanisms that can lead to anaphylactic or severe allergic reactions, and some may not be preventable despite product modification.
- Even in the presence of low IgA levels and anti-IgA antibodies, risk of anaphylactic transfusion reaction is low.
- Anti-IgA testing is only recommended in selected individuals with history of one anaphylactic transfusion reaction or two severe allergic transfusion reactions.
- The form for ordering anti-IgA testing can be found on the Canadian Blood Services website.
- Instructions and guidance on how to report adverse transfusion reactions can be found in this guide on the Canadian Blood Services Professional Education website.
Anaphylactic transfusion reactions
Definition
The International Society of Blood Transfusion (ISBT) and International Hemovigilance Network (IHN) define anaphylactic reactions as mucocutaneous symptoms (itchy rash, hives, swelling of the lips, redness and swelling around the eyes and of the conjunctiva) accompanied by upper or lower airway obstruction and/or severe hypotension occurring during or shortly after transfusion.1 Reactions are often life-threatening. Allergic reactions are considered severe in the presence of the following clinical findings: hypotension, dyspnea or cough, tachycardia, generalized flushing or anxiety, nausea or vomiting, widespread urticaria covering over two-thirds of body surface area.2,3,4,5 For severe allergic reactions, different hemovigilance systems have slightly different definitions.1,6,7
Incidence
The incidence of anaphylactic transfusion reactions varies in different hemovigilance schemes, depending on the precise definition used. In general, the incidence is higher in blood components containing a high volume of plasma. Between 2009 to 2013, 5.9 severe allergic reactions per 100,000 components transfused were reported to Public Health Agency of Canada Transfusion Transmitted Injuries Surveillance System (TTISS).8 This is an overestimate of the true risk of severe allergic reactions as it includes cases that were eventually found to have a doubtful relationship to transfusion. In 2018, the Dutch hemovigilance system (TRIP) reported 58 anaphylactic reactions out of 494,720 components transfused (1.2 per 100,000 components).6 In the U.K., the Serious Hazards of Transfusion (SHOT) hemovigilance program reported 166 severe allergic reactions from 2016 to 2019 inclusive, out of over 9.5 million blood components (red blood cells, platelets, frozen plasma, solvent-detergent plasma, and cryoprecipitate) distributed to hospitals (1.5 per 100,000 components).7
In summary, severe allergic or anaphylactic reactions are reported in 1.2 to 5.9 per 100,000 components transfused in various hemovigilance systems. Reactions may be underreported and not all are fully investigated for IgA deficiency and the presence of anti-IgA.
Clinical presentation
An anaphylactic or severe allergic reaction may begin after infusion of a small volume of the blood component with symptoms that may be initially mild, but can rapidly progress to loss of consciousness, distributive shock, and, in rare cases, death. Reactions usually occur within 1– 45 minutes of the start of transfusion, although the less severe allergic reactions may begin up to 2–3 hours after the transfusion was initiated.1 Anaphylactic or severe allergic transfusion reaction can be differentiated from other serious transfusion reactions such as those due to bacterial contamination or acute hemolytic transfusion reactions, by the presence of cutaneous symptoms, the absence of fever and chills, and the presence of severe respiratory symptoms. Symptoms and signs of anaphylactic transfusion reactions are listed in Table 1.
| Cutaneous |
|
| Pulmonary |
|
| Gastrointestinal |
|
| Cardiovascular |
|
| Miscellaneous |
|
Mechanism
Although incompletely understood, the mechanisms of anaphylactic transfusion reactions include9:
- Presence of an allergen in the donated blood component for which the recipient is sensitized
- Pre-formed IgE from blood donor against allergens that that recipient may have been exposed to
- Biologic response modifiers, such as C3d, that accumulate during storage of blood components
- Incidental and concomitant exposure of the transfusion recipient to another allergen
IgE-mediated reactions are type I immediate hypersensitivity reactions. IgE can bind to mast cells and tissue basophils through Fc receptors on their cell membranes. Mast cells (armed with a surface coating of IgE) are triggered when the patient next becomes exposed to the antigen (allergen) recognized by the IgE. This leads to cross-linking of the bound IgE antibody, producing mast cell degranulation with release of preformed and new mediators of the allergic reaction.
Histamine is the most important preformed mediator. It causes vasodilation, increased vascular permeability, increased mucous secretion by nasal and bronchial glands, and smooth muscle contraction. Other granule compounds that mediate the reaction include heparin, enzymes, leukotrienes, cytokines, and activating factors such as the eosinophil and neutrophil chemotactic factors and platelet activating factor.
In most cases, the exact cause is unknown. Often, patients receiving transfusion may also concomitantly be exposed to other medications or food items that might have caused the reaction. There are rare case reports of passive transfer of donor IgE antibodies against peanuts causing an anaphylactic reaction in recipients who subsequently ingested peanuts.There has also been a case of transfer of the peanut antigen by transfusion to a recipient with a severe peanut allergy. Recipient atopic predisposition, as demonstrated by higher total IgE and IgE specific for aeroallergens such as pollens, has been associated with increased allergic reactions on receiving blood in general, but not necessarily with anaphylactic reactions.3,12
Haptoglobin deficiency
Moreover, recipients may have a deficiency of serum proteins or different allotypes of serum proteins, such as haptoglobin. Haptoglobin deficiency is more common is certain ethnicities, such as Han Chinese (1 in 1,000), Koreans (1 in 1,500), and Japanese (1 in 4,000).10 In Japan, 1.6% of anaphylactic transfusion reactions are due to anti-haptoglobin antibodies in individuals with haptoglobin deficiency.11
IgA deficiency
IgA deficiency is the most common human immunodeficiency. Although most IgA deficient individuals are asymptomatic, some IgA deficient individuals have a higher prevalence of respiratory and gastrointestinal tract infections. From a transfusion medicine perspective, the presence of anti-IgA in an IgA deficient recipient is a possible cause of anaphylactic transfusion reactions. Approximately 1 to 5% of anaphylactic transfusion reactions in a Caucasian population may be associated with anti-IgA in IgA deficient recipients.3,13
Investigation of patients with anaphylactic transfusion reactions
If a patient develops an anaphylactic transfusion reaction, a comprehensive assessment should be performed to assess both transfusion-related and unrelated causes of anaphylaxis.
Depending on the clinical scenario, screening tests may include immunoglobulin quantification for IgA levels as well as haptoglobin levels. If, based on immunoglobulin quantification, the patient appears to be IgA deficient, samples should be sent to Canadian Blood Services to determine if anti-IgA is present.
IgA level interpretation
IgA levels between approximately 100 and 600 mg/dL1 are normal. However, many individuals with lower IgA levels are not truly deficient. Commonly used immunoglobulin quantification assays may be used as a screening test to identify individuals with an IgA level below 2 to 4 mg/dL. A more sensitive enzyme-linked immunosorbent assay (ELISA) method with a sensitivity of 0.02 mg/dL is required to determine which individuals are truly IgA deficient. Only those with true IgA deficiency (i.e. levels below 0.05 mg/dL) may develop anti-IgA antibodies.
At Canadian Blood Services, screening of approximately 100,000 donor samples identified 139 donors with confirmed IgA levels <0.05 mg/dL.14,15 Based on the number of individual donors the rate of severe IgA deficiency in our donors is approximately 1 in 300. At Héma-Québec, 73 IgA-deficient blood donors were identified from close to 39,000 screened donor samples, for a frequency of 1 in 531.16
Frequency and clinical significance of anti-IgA antibodies
Blood donors
In the Canadian Blood Services donor screening program, anti-IgA antibodies were identified in 41% of severely deficient donors.15 In the Héma-Québec donor screening program, anti-IgA antibodies were found in 53% of severely deficient donors.5 Many anti-IgA antibodies are naturally occurring. Among Héma-Québec donors, only 9 of the 39 donors with antibodies had a history of transfusion or pregnancy.
Recipients with anaphylactic transfusion reactions
At Canadian Blood Services, from 0 to 4 anaphylactic transfusion reactions that occur each year are likely related to anti-IgA antibodies. In 2015, none of the 12 patients investigated for anaphylactic transfusion reactions were found to have anti-IgA; in 2016, 1 of 9 patients investigated was found to have anti-IgA.17 At Héma-Québec, from 0 to 1 anaphylactic reactions related to anti-IgA are reported yearly.18 In the U.K. SHOT database, from 2010 to 2015 (6 years), out of 120 cases of acute transfusion reactions that reported IgA testing results, anti-IgA was found in 8 (personal communication, Paula-Bolton-Maggs, November 2016).7 In the TRIP database,6 between 2003 to 2018, only 5 (0.7%) severe allergic or anaphylactic reactions were found to occur in individuals with IgA deficiency and anti-IgA antibodies.
If the frequency of IgA deficiency and anti-IgA is similar in recipients and in donors, one would expect that approximately 1 to 1.6 patients in 1,000 in Canada would be IgA deficient with anti-IgA. However, the frequency of anaphylactic transfusion reactions related to anti-IgA is less than 1 in 500,000 components transfused annually. The vast majority of anti-IgA antibodies do not appear to cause anaphylactic transfusion reactions. Moreover, a review of a French hemovigilance database found that 12 patients with a history of anaphylaxis with absolute IgA deficiency and anti-IgA antibodies, subsequent transfusion provoked allergic reaction in only one patient.19 In a study of 229 patients with allergic transfusion reactions, only 1.3% had an absolute IgA deficiency and anti-IgA antibodies.20 Hence, even in the presence of anti-IgA antibodies in the recipient, risk of severe allergic or anaphylactic transfusion reaction is low.
Testing patients for anti-IgA antibodies
Individuals with a history of an anaphylactic transfusion reaction or two severe allergic reactions may benefit from further IgA level and anti-IgA testing. However, the utility of performing testing on patients who appear to have low IgA levels but have not been transfused, or have not had a transfusion reaction is unclear.21-23 Given the high frequency of anti-IgA antibodies and the low frequency of anaphylactic transfusion reactions attributable to anti-IgA, it is estimated that approximately 1 in 100 patients with IgA deficiency and anti-IgA antibodies develop transfusion reactions. There is no available test to distinguish which anti-IgA antibodies may be of clinical significance. A positive result in patients where anti-IgA testing is not indicated may result in unnecessary delays in transfusion due to uncertainty about the requirements for specialized products, or clinical hesitation to transfuse due to fear of a transfusion reaction. Therefore, the majority of practitioners do not recommend testing of patients who are low in IgA for the presence of anti-IgA, in the absence of a history of an anaphylactic (or 2 severe) transfusion reactions.21 Moreover, if previous testing for IgA has shown detectable levels and/or previous anti-IgA testing has shown no detectable anti-IgA antibodies, further testing for anti-IgA is not recommended.
At Canadian Blood Services, anti-IgA testing may be considered in the following patients (see Figure 1):
- A patient with a history of an anaphylactic transfusion reaction and markedly decreased or absent IgA.
- A patient with a history of two or more severe allergic transfusion reactions, not quite meeting definition of anaphylaxis. https://www.blood.ca/en/requisitions-and-forms
Canadian Blood Services no longer performs IgA quantitative studies prior to sending samples for anti-IgA testing. The identification of IgA deficiency through hospital-based testing is recommended. For anti-IgA testing, hospital laboratories send a pre-transfusion frozen patient serum (red top or SST tube) sample to Canadian Blood Services. This sample is then sent to an external laboratory for testing. The test is an ELISA performed on the serum with a required minimum volume of 0.5 ml to detect human polyclonal anti-IgA antibodies. Frozen specimens must be tested within 30 days of freezing. The test is considered positive if anti-IgA antibodies at a ≥99 U/ml concentration are detected. The anticipated turnaround time for this test may be up to 15 days.
To order anti-IgA testing, please use the standardized order form available on the Canadian Blood Services website at: https://www.blood.ca/en/requisitions-and-forms
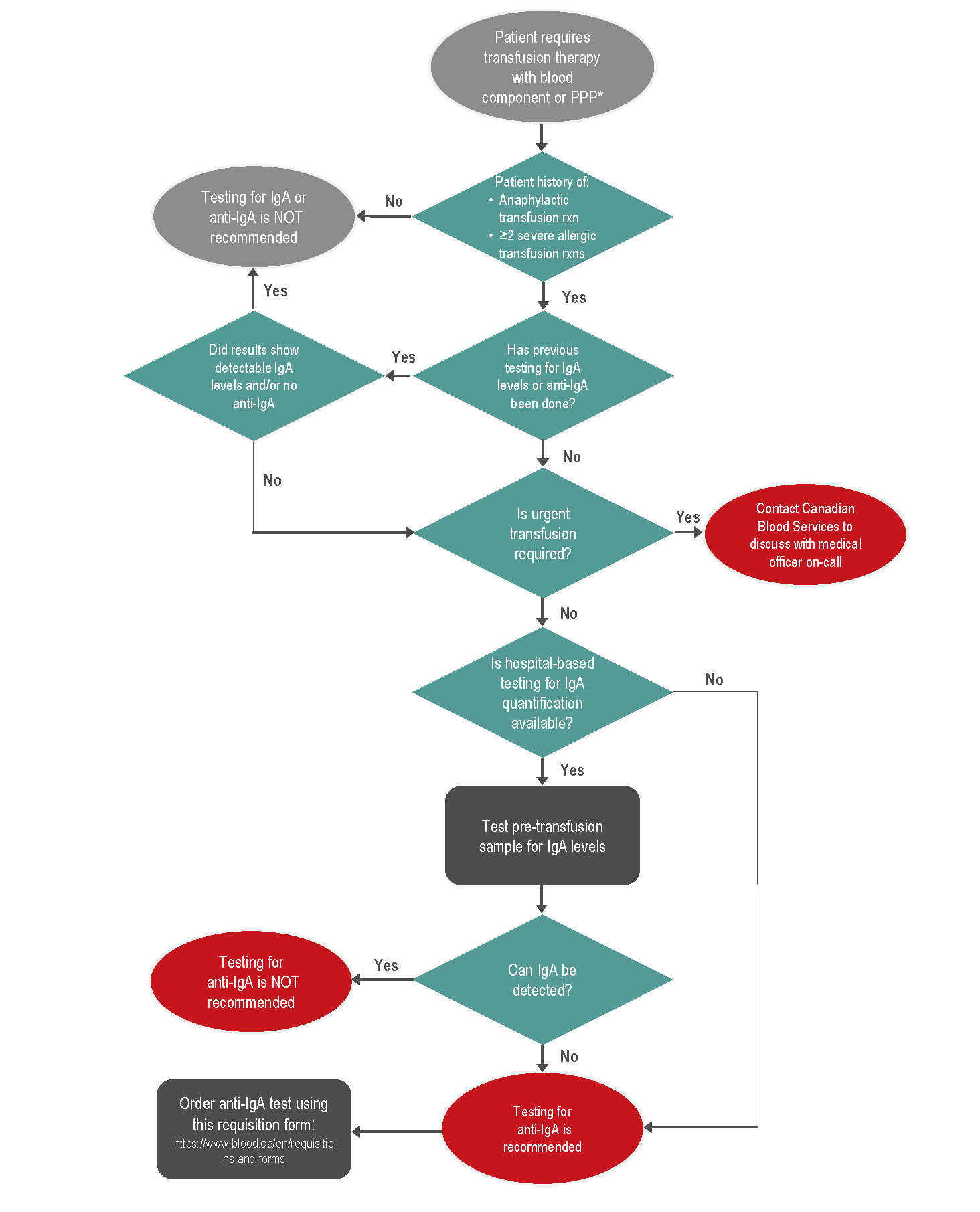
Figure 1: Clinical algorithm to aid decision making on IgA and anti-IgA testing for patients requiring transfusion therapy for customers of Canadian Blood Services.
* PPP = plasma protein product
Transfusion support for patients with a history of anaphylactic transfusion reactions
For patients with a history of anaphylactic transfusion reactions, ideally, an investigation would be done to determine if anti-IgA antibodies are present. Transfusion management of patients with a history of an anaphylactic transfusion reaction and anti-IgA antibodies is outlined in Table 2. If the patient is not IgA deficient and/or no anti-IgA has been detected, and the patient has experienced only a single anaphylactic reaction, a trial transfusion of unwashed blood components may be performed under controlled conditions, including patient consent, premedication, and close medical supervision. If an anaphylactic reaction occurs again, the patient should be transfused using components washed to remove the maximal amount of plasma.3,24 For red blood cells, a double wash (also called extra wash) protocol is necessary to adequately reduce IgA levels to less than 0.05 mg/dL using an automated cell processor (See Chapter 15 of the Clinical Guide to Transfusion for more on washed blood components).24 If there is inadequate time to perform testing for anti-IgA, the case should be discussed with a Canadian Blood Services medical officer.
If haptoglobin deficiency is determined to be the etiology of the anaphylactic (or 2 severe) allergic transfusion reaction, specialized products may be required for transfusion support. For red cells, washed red cells may be used. However, for platelets and plasma, products may need to be imported from international blood centres that have haptoglobin-deficient donors in their registry. The process of importation of specialized blood products can take several weeks and these cases should be reviewed with a Canadian Blood Services Medical Officer on-call or the Rare Blood Program.
| Red Blood Cells (RBCs) |
|
| Platelets |
|
| Fresh Frozen Plasma, Frozen Plasma |
|
| Cryoprecipitate |
|
| Plasma Derivatives (IVIg, Albumin, Rh Immune Globulin) |
|
Canadian Blood Services Resources
- Customer Letter issued January 22, 2018 (available on blood.ca)
- Test for Anti-IgA (Frequently Asked Questions) (available on blood.ca)
- Patient Request for Anti-IgA Testing, Form F800014 (available on blood.ca)
- Clinical algorithm. Print out the algorithm to aid decision making on IgA and anti-IgA testing for patients requiring transfusion therapy.
Suggested citation
Khandelwal A, Clarke G, Goldman M. Anaphylactic transfusion reactions and IgA deficiency [Internet]. Ottawa: Canadian Blood Services; 2021 [cited YYY MM DD]. Available from: professionaleducation.blood.ca/en/transfusion/publications/anaphylactic-transfusion-reactions-and-iga-deficiency
References
1. International Society of Blood Transfusion-Working Party on Haemovigilance. Appendix B, Proposed Standard Definitions for Surveillance of Non Infectious Adverse Transfusion Reactions. In Hemovigilance: An Effective Tool for Improving Transfusion Safety. Edited by De Vries R, Faber J. Published by John Wiley & Sons, Ltd., 2011. https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781118338179.app2.
2. Callum JL, Pinkerton PH, Lima A, Lin Y, Karkouti K, Lieberman L, Pendergrast JM, Robitaille N, Tinmouth AT, Webert KE, . Bloody Easy 4: Blood Transfusions, Blood Alternatives and Transfusion Reactions. Published in Ontario, Canada by Ontario Regional Blood Coordinating Network, 2016. http://transfusionontario.org/en/download/bloody-easy-4-blood-transfusions-blood-alternatives-and-transfusion-reactions-a-guide-to-transfusion-medicine-fourth-edition/.
3. Vamvakas E. Allergic and Anaphylactic Reactions. In Transfusion Reactions, 4th Edition Edited by Popovsky M. Published in Arlington, VA by AABB Press 2012.
4. Canadian Blood Services. Adverse Reactions Reporting. Canadian Blood Services, 2020. https://professionaleducation.blood.ca/en/transfusion/publications/adverse-reactions-reporting.
5. Callum J, Pinkerton P, Lima A, Lin Y, Karkouti K, Lieberman L, Pendergrast J, Robitaille N, Tinmouth A, Webert K. Adverse Reactions. in Clarke G, Charge S, (eds) Clinical Guide to Transfusion. ProfessionalEducation.blood.ca, Canadian Blood Services, 2017. https://professionaleducation.blood.ca/en/transfusion/clinical-guide/adverse-reactions.
6. TRIP Foundation. Trip Report 2018, Hemovigilance - Extended Version. 2018: p. 40. https://www.tripnet.nl/wp-content/uploads/2020/08/Trip.HEMO_uitgebreid_ENGdef2020-4.pdf.
7. Serious Hazards of Transfusion-SHOT. Annual Shot Reports. https://www.shotuk.org/shot-reports/.
8. Public Health Agency of Canada. Transfusion Transmitted Injuries Surveillance System (Ttiss): 2009-2013 Summary Results. Centre for Communicable Diseases and Infection Control, Public Health Agency of Canada2016. http://www.healthycanadians.gc.ca/publications/drugs-products-medicaments-produits/blood-transfusion-2013-transfusionnels/index-eng.php (Last accessed January 8 2021).
9. Yasui K, Matsuyama N, Takihara Y, Hirayama F. New Insights into Allergic Transfusion Reactions and Their Causal Relationships, Pathogenesis, and Prevention. Transfusion 2020; 60: 1590-601. https://onlinelibrary.wiley.com/doi/abs/10.1111/trf.15845.
10. Shimada E, Tadokoro K, Watanabe Y, Ikeda K, Niihara H, Maeda I, Isa K, Moriya S, Ashida T, Mitsunaga S, Nakajima K, Juji T. Anaphylactic Transfusion Reactions in Haptoglobin-Deficient Patients with Ige and Igg Haptoglobin Antibodies. Transfusion 2002; 42: 766-73. https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1537-2995.2002.00117.x.
11. Koda Y, Watanabe Y, Soejima M, Shimada E, Nishimura M, Morishita K, Moriya S, Mitsunaga S, Tadokoro K, Kimura H. Simple Pcr Detection of Haptoglobin Gene Deletion in Anhaptoglobinemic Patients with Antihaptoglobin Antibody That Causes Anaphylactic Transfusion Reactions. Blood 2000; 95: 1138-43.
12. Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic Transfusion Reactions to Platelets Are Associated More with Recipient and Donor Factors Than with Product Attributes. Transfusion 2011; 51: 1716-22. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1537-2995.2010.03009.x.
13. Sandler SG, Eder AF, Goldman M, Winters JL. The Entity of Immunoglobulin a-Related Anaphylactic Transfusion Reactions Is Not Evidence Based. Transfusion 2015; 55: 199-204. https://www.ncbi.nlm.nih.gov/pubmed/25066014.
14. Palmer DS, O'Toole J, Montreuil T, Scalia V, Yi QL, Goldman M, Towns D. Screening of Canadian Blood Services Donors for Severe Immunoglobulin a Deficiency. Transfusion 2010; 50: 1524-31. https://www.ncbi.nlm.nih.gov/pubmed/20158683.
15. Palmer DS, O'Toole J, Montreuil T, Scalia V, Goldman M. Evaluation of Particle Gel Immunoassays for the Detection of Severe Immunoglobulin a Deficiency and Anti-Human Immunoglobulin a Antibodies. Transfusion 2012; 52: 1792-8. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1537-2995.2011.03513.x.
16. Thibault L, Beauséjour A, De Grandmont MJ, Long A, Goldman M, Chevrier M-C. Establishment of an Immunoglobulin a–Deficient Blood Donor Registry with a Simple in-House Screening Enzyme-Linked Immunosorbent Assay. Transfusion 2006; 46: 2115-21. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1537-2995.2006.01037.x.
17. O'Brien S. Surveillance Report 2019, Canadian Blood Services. https://professionaleducation.blood.ca/en/transfusion/publications/surveillance-report (Last accessed May 3 2021).
18. Héma-Québec. Rapport Du Comité D’hemovigilance,2008. https://publications.msss.gouv.qc.ca/msss/fichiers/2009/09-212-05.pdf (Last accessed January 8 2021).
19. Tacquard C, Boudjedir K, Carlier M, Muller J-Y, Gomis P, Mertes PM. Hypersensitivity Transfusion Reactions Due to Iga Deficiency Are Rare According to French Hemovigilance Data. Journal of Allergy and Clinical Immunology 2017; 140: 884-5. https://doi.org/10.1016/j.jaci.2017.03.029.
20. Winters JL, Moore SB, Sandness C, Miller DV. Transfusion of Apheresis Plts from Iga-Deficient Donors with Anti-Iga Is Not Associated with an Increase in Transfusion Reactions. Transfusion 2004; 44: 382-5. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1537-2995.2003.00662.x.
21. Sandler SG. How I Manage Patients Suspected of Having Had an Iga Anaphylactic Transfusion Reaction. Transfusion 2006; 46: 10-3. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1537-2995.2006.00686.x.
22. Lilic D, Sewell WAC. Iga Deficiency: What We Should—or Should Not—Be Doing. Journal of Clinical Pathology 2001; 54: 337-8. https://jcp.bmj.com/content/jclinpath/54/5/337.full.pdf.
23. Sandler SG, Vassallo RR. Anaphylactic Transfusion Reactions. Transfusion 2011; 51: 2265-6. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1537-2995.2011.03404.x.
24. Hansen AL, Turner TR, Kurach JD, Acker JP. Quality of Red Blood Cells Washed Using a Second Wash Sequence on an Automated Cell Processor. Transfusion 2015; 55: 2415-21. http://www.ncbi.nlm.nih.gov/pubmed/25988774.