Impact of COVID-19 on blood donation in Canada
Author: Aditi Khandelwal, MDCM, FRCPC
Blood transfusion is a crucial component of medical therapy today. Having a resilient blood system that can withstand widespread crises, such as pandemics, is an important feature of a sustainable blood supply.1 Pandemics are a sustained disaster with significant impact on the supply of labile components (e.g., red blood cells, platelets, plasma) and non-labile products (e.g., immune globulins). SARS-CoV-2, the virus responsible for COVID-19 illness, is not transmissible by blood (see Canadian Blood Service’s COVID-19 FAQ for health professionals working with blood products). However, COVID-19 had an impact on donor eligibility, donor availability, and availability of trained personnel to collect and produce blood products.2,3
Donors are a vital part of the blood system. Their selfless donations ensure that individuals in need of labile blood components across the country are well supported. Different types of donation include whole blood donation, apheresis plasma or platelet donation, organ donation, stem cell donation and cord blood donation. Here, we will review the impact of the COVID-19 pandemic on whole blood donations. For more on how whole blood donations are separated into specific cellular (red blood cells and platelets) and plasma components, see Chapter 2 of Canadian Blood Services’ Clinical Guide to Transfusion.
Donor attendance
During the first wave of COVID-19, enhanced safety protocols were implemented at donor sites. Community donor events were cancelled due to fear, logistical challenges, and the time needed to implement safety protocols. Enhanced cleaning, social distancing and wellness checkpoints decreased donor throughput. For safety and to accommodate application of required safety measures, drop-ins were eliminated and pre-booked appointments became mandatory.
Between February 2020 and May 2020, there was a decline in collections due to both donor attendance and decreased capacity. Pre-pandemic, donation centres accommodated over 70,000 donors per month. By May 2020 only 54,738 donors attended a donor centre, with recovery to a robust 72,853 donors by December 2020 (Figure 1). This corresponded to a decrease in total whole blood collections: the pre-pandemic baseline of approximately 200,000 whole blood collections per quarter decreased by the second quarter of 2020 to a nadir of around 160,000 collections. Whole blood collections improved by the fourth quarter of 2020, nearing pre-pandemic levels at over 190,000 collections.5
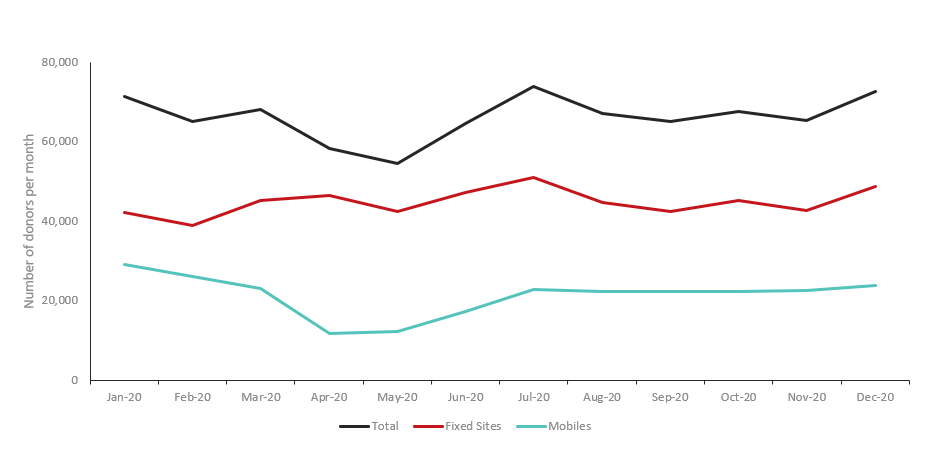
Figure 1: Donor attendance by month in 2020 for whole blood collection events at permanent sites (n=34) and mobile clinics (n=480).
Early in the pandemic, in cities across Canada, only 58 % of available appointment slots were filled, on average. Efforts were made to improve recruitment. Direct phone calls were made to donors and public service announcements were made by prominent leaders, including Prime Minister Justin Trudeau. By December 2020, donor attendance improved and 79% of available slots were filled.
There were clear geographic differences in the proportion of appointment slots that were filled. Initially, both larger and smaller urban centres had over 40% of their collection appointments filled. By December, the proportion of unfilled appointments were highest in cities impacted most by COVID-19. For instance, in Toronto, one of the cities with highest number of COVID-19 cases, unfilled collection appointments peaked at 51.5% in February 2020, then decreased to 39.2% by December 2020 (Figure 2). Whereas, in Calgary, Edmonton and Vancouver, the proportion of unfilled appointments dropped to less than 20% by December 2020.
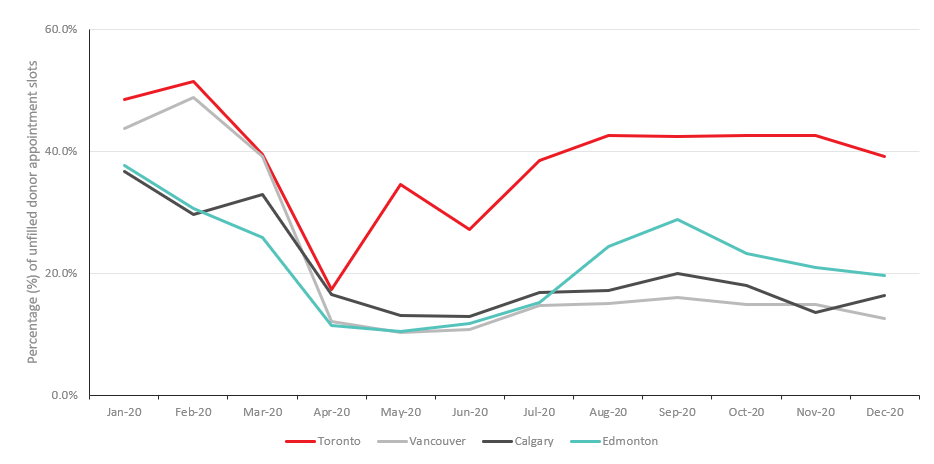
Figure 2: Percentage of unfilled donation appointments in four major Canadian cities.
During the pandemic, Canadian Blood Services shifted to having more blood collection events at their permanent sites. Across Canada, Canadian Blood Services has 34 permanent and 480 mobile whole blood collection sites. Pre-pandemic, 52% of the blood was being collected at permanent sites.4 Permanent sites offer fewer logistical challenges related to clinic flow, transportation and implementation of enhanced safety protocols. As a direct consequence of the pandemic, 2,248 collection events were cancelled, with mobile events disproportionately affected (Figure 3). By December 2020, 69% of whole blood was being collected at permanent sites.5
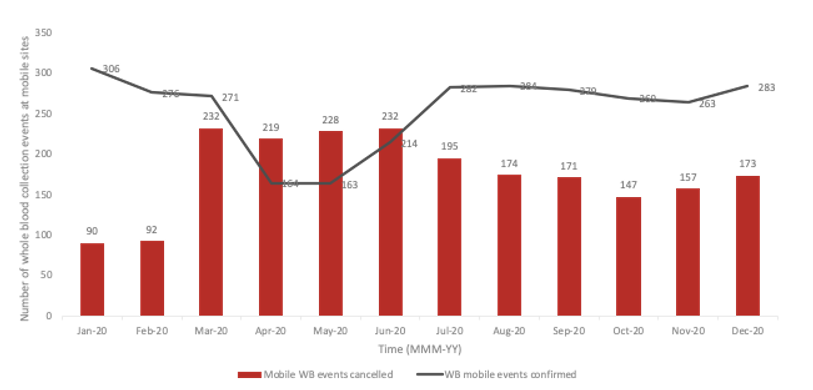
Figure 3: Whole blood events that were confirmed or cancelled at mobile sites during the pandemic.
Donor selection criteria
In Canada, there are two organizations tasked with collection, manufacturing and distribution of blood products: Canadian Blood Services and Héma-Québec. These organizations are not-for-profit, non-governmental agencies, funded by federal, provincial and territorial governments and regulated by Health Canada. For an overview of the donor selection process, see Chapter 6 in Canadian Blood Services’ Clinical Guide to Transfusion.
Both Canadian Blood Services and Héma-Québec adapted their donor assessment policies and limited close interactions between donors and frontline collection staff. Wellness checks, including temperature measurements, were established at the entrance of donor centres and the requirement for blood pressure measurements were removed to minimize close contact between frontline staff.5
Key changes in donor selection criteria implemented during the pandemic are described in Table 1. By reducing the hemoglobin threshold acceptable for donation from 125g/L to 120g/L for women and from 130g/L to 125g/L for men, overall deferrals for anemia (i.e., low hemoglobin) fell from 3.4% (January 2020) to 1.7% (August 2020) overall, with a significant decrease in deferrals for women from about 6.1% in January 2020 to 2.7% in August 2020 when this criterion was applied. This allowed an additional 1,000 women to be able to donate per month. In October 2020 the hemoglobin criteria reverted to pre-pandemic criteria as no shortages in labile products were observed. For more on the importance of iron for whole blood donors, see this Canadian Blood Services publication.
A reduction in the number of days a person would be deferred from donating after travel to malaria endemic regions led to a reduction in donor deferral by 3% (from 6.1% to 3.1%), leading to a gain of approximately 2,000 donations per month. The travel criteria changes will likely require close assessment once rates of vaccination increase and travel bans are lifted.
Table 1: Major changes to key donor criteria in 2020.
| Criteria | Pre-pandemic | Changes during pandemic |
|---|---|---|
| Hemoglobin level |
|
|
| Travel |
|
|
| COVID-19 vaccination | n/a | No deferral required. |
| COVID-19 | n/a |
|
In summary, donors demonstrated ongoing commitment to blood donation during the pandemic. Blood centres adapted to the necessary public health measures and maintained safe donation sites. Together, Canada’s blood supply demonstrated resilience and was able to meet patient demands.
Suggested citation
Khandelwal A. Impact of COVID-19 on blood donation in Canada [Internet]. Ottawa: Canadian Blood Services; 2021 [cited YYY MM DD]. Available from: https://professionaleducation.blood.ca/en/transfusion/publications/impact-covid-19-blood-donation-canada
References
- Mulcahy AW, Kapinos KA, Briscombe B, et al. Towards a sustainable blood supply in the United States: An analysis of the current system and alternatives for the future. RAND Corporation. 2016: Santa Monica - Chapters 7 and 9.
- Yazer MH, Jackson B, Pagano M, et al. Vox Sanguinis International forum on hospital transfusion services’ response to COVID-19: Responses. Vox Sang. 2020;115:e1-e17.
- Stanworth S, New HV, Apelseth TO, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7:e756-64.
- Shaman J. Pandemic preparedness and forecast. Nat Microbiol. 2018; 3(3):265-267.
- Sher, G. Open Board Meeting, December 1 – 3, 2020 accessible at: https://www.blood.ca/sites/default/files/CEO_Mid_Year_Review_Q1-Q2_2020-2021_-_OBM_Presentation.pdf