Chapter 18
Platelet transfusion, alloimmunization and management of platelet refractoriness
Background
Platelets are protein rich fragments with a diameter of two to three microns and no nucleus.1 Their main function is to mediate primary hemostasis, though they also play a critical role in primary immunity, tumor progression and inflammation.2 Platelets circulate in the blood stream until they are exposed to the subendothelial matrix following an injury to a blood vessel, at which point the platelets activate and undergo morphologic changes. Once activated, platelets bind to the sites of injury and to each other to form a temporary hemostatic plug. This initiates the activation of additional plasma coagulation factors to form a more permanent fibrin hemostatic plug.
A normal platelet count is 150–400 x 109 per litre; individuals with platelet counts of less than 10 x 109 per litre are at an increased risk of spontaneous and serious bleeding. There is a risk of bleeding complications from surgery or other injuries that can increase with platelet counts below 30–50 x 109 per litre. Individuals with congenital or acquired disorders of platelet function also have an increased risk of bleeding.
Platelet transfusions can be used to increase the number of functional platelets and therefore decrease the risk of bleeding. This chapter describes the process of collecting, manufacturing and storing platelets for transfusion, summarizes clinical practice recommendations for platelet transfusions and provides information on adverse reactions and platelet refractoriness.
Collection, manufacturing, and storage of platelets for transfusion
Platelet concentrates are collected and concentrated using two main methods:
- Buffy coat method: With this method, whole blood is collected and spun to separate the platelets from the plasma and the red blood cells (illustrated in Chapter 2, Blood components, in this Guide). Following this separation, multiple units of platelets from group-identical donors are pooled together and suspended in either plasma or a platelet additive solution (PAS). This is often referred to as “random donor” or pooled platelets.
- Apheresis method: With this method, platelets are collected from a single donor using an apheresis machine. The machine extracts whole blood from the donor, spins it to separate the platelets and a small amount of plasma, and returns the rest of the blood components back to the circulating blood of the donor.
Of note, platelets collected either by whole blood or apheresis methods can be further modified. These modifications include use of pathogen inactivation technology (see Chapter 19, Pathogen-reduced platelets in this Guide), suspension in platelet additive solution E (PAS-E) rather than plasma, and testing for anti-A and anti-B titres and labelling as low-titre. Platelet pools are labeled as “Low anti-A/B” only when all the donor units within the pool were found to have anti-A and anti-B levels below a predetermined cut-off (see our FAQ: Donor high titre isohemagglutinin (anti-A, anti-B) testing at Canadian Blood Services).
Summarized information on platelet component characteristics (i.e., mean volume, number of donors in a unit, mean plasma volume, approximate platelet yield, resuspension solution, type of anticoagulant, bacterial screening process, and component shelf-life) can be found in Table 2 of Chapter 19 of this Guide.
Platelets derived from both apheresis and buffy coat manufacturing processes are leukoreduced and tested for bacterial growth (unless pathogen reduced; bacterial testing is not required for pathogen-reduced platelets) and are considered to be equally effective (See Chapter 2, Blood components, in this Guide). The main indication for apheresis platelets is the provision of platelets for patients with documented alloantibodies targeting human leukocyte antigen (anti-HLA) or human platelet antigen (anti-HPA) and alloimmune platelet refractoriness, or in the setting of post-transfusion purpura or neonatal alloimmune thrombocytopenia.
Platelet units are stored at room temperature with agitation and have a seven-day shelf life. See Chapter 2, Blood components, for more information on platelet storage.
Canadian Blood Services protocols and procedures determine the acceptability of platelet units for distribution. Although Canadian Blood Services uses strict protocols to manufacture blood components, the components may be exposed to conditions that alter their appearance after manufacturing, such as during distribution or storage. Whether or not a platelet unit is suitable for transfusion after distribution is determined by local hospital policy and procedures. The Visual Inspection Tool is an educational resource meant to inform the user about visual variability of blood components and its causes and can be used in conjunction with other required protocols or work instructions for the visual inspection of blood components. For a visual comparison of platelet types manufactured by Canadian Blood Services, see Chapter 19 of this Guide and for additional images of platelet units next to conditions or characteristics that may affect their visual appearance, see the Visual Inspection Tool.
Indications in adult patients
Platelets are transfused into individuals with thrombocytopenia or platelet dysfunction for two indications:
- to stop bleeding (therapeutic platelet transfusions)
- to prevent bleeding (prophylactic platelet transfusions)
Transfusion of one unit of platelets is expected to increase the platelet count of a 70 kg adult by 15–25 x 109 per litre;3 however, the increment may be more or less depending on the underlying cause of the thrombocytopenia, comorbidities, and patient size.
Therapeutic platelet transfusions
There is limited high-quality evidence to guide the use of platelet transfusions to treat bleeding. However, there is general agreement about the clinical indications for platelet transfusions in the context of bleeding (see Table 1).
It is important to consider the underlying etiology of the thrombocytopenia or platelet dysfunction and the bleeding site. Thrombocytopenia can be caused by decreased production or by increased destruction, consumption, or sequestration of platelets. In the case of thrombotic thrombocytopenic purpura (TTP) and heparin-induced thrombocytopenia (HIT), platelet transfusions are generally avoided as they may be harmful and increase the risk of thrombotic events. In the case of immune thrombocytopenic purpura (ITP), platelet transfusions are generally reserved for critical bleeding since the platelet count increment is attenuated in patients with ITP and risks of platelet transfusions may outweigh benefits in most cases.
Additional hemostatic agents shall be considered where appropriate, in the case of major active bleeding. Antifibrinolytics such as tranexamic acid are especially useful for mucosal bleedings (e.g., oral, gastrointestinal, gynecologic). Tranexamic acid has also been associated with better survival in the context of bleeding trauma patients,4 post-partum hemorrhage,5 and traumatic brain injury.6
Specific anticoagulant reversal (e.g., protamine for heparin, idarucizumab for dabigatran), or use of prothrombin complex concentrates (PCCs) and vitamin K for warfarin reversal is valuable and should be considered.
The efficacy and adjunct use of desmopressin (DDAVP) is being increasingly questioned. Although in animal models DDAVP may improve platelet function, there has been limited evidence of benefit for patients with uremia, especially pre-kidney biopsy,7 or those on antiplatelet agents8 and/or awaiting surgical intervention.9 DDAVP may be of benefit in select patients with von Willebrand disease. (see Chapter 17, Hemostatic disorders and hereditary angioedema).10 DDAVP use requires close monitoring and the clinical team to balance the risks of hyponatremia, hypertension, thrombosis, and tachyphylaxis.
Table 1: Summary of clinical practice recommendations† for therapeutic platelet transfusions in bleeding adult patients
| Clinical setting | Recommendation |
|---|---|
| Significant bleeding | Transfuse one dose if the platelet count is less than 50 x 109/L and check platelet count again. |
| Head trauma or central nervous system hemorrhage | Transfuse one dose if the platelet count is less than 100 x 109/L and check the platelet count again. |
| Platelet dysfunction and significant bleeding (e.g., ASA, P2Y12 therapy) | Consider transfusing one dose at any platelet count to treat the antiplatelet effect. |
| Treatment of spontaneous intracranial hemorrhage in patients on acetylsalicyclic acid (ASA) and/or P2Y12 therapy | Platelet transfusions not recommended. Literature suggests no clinical benefit, but possible harm in patients with spontaneous intracranial hemorrhage on anti-platelet therapy.11 |
| Platelet dysfunction and significant bleeding associated with cardiopulmonary bypass | Consider transfusing one dose; however, standard platelet transfusion may be associated with increased adverse events and may not be beneficial. |
| Bleeding in a patient with immune thrombocytopenia (ITP) |
Consult a hematologist. Might consider transfusing one dose for life-threatening bleeding only if platelet count is below 20 x 109/L. |
| Bleeding trauma patients | Use platelet transfusions as part of a massive transfusion protocol. |
†Clinical practice recommendations were compiled from a review of evidence-based guidelines, Choosing Wisely and Choosing Wisely Canada lists, and current literature. Because a formal literature search was not part of the preparation of these recommendations, they are presented as recommendations rather than guidelines.
Prophylactic platelet transfusions
The Association for Advancement of Blood and Biotherapies (AABB), the International Collaboration for Transfusion Medicine Guidelines (ICTMG), the American Association of Clinical Oncology (ASCO) and the British Committee for Standards in Hematology (BCSH) conducted separate literature reviews using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) method to assess the quality of evidence for prophylactic platelet transfusions. The resulting clinical practice guidelines12-16 make a number of recommendations for the use of platelet transfusions to prevent bleeding.
We compiled clinical practice recommendations for prophylactic platelet transfusions from a review of these evidence-based guidelines, as well as Choosing Wisely and Choosing Wisely Canada, the current literature, and expert opinion. We summarized these recommendations in Table 2.
Table 2: Summary of clinical practice recommendations for prophylactic platelet transfusions in adult patients
| Clinical setting | Recommendation | Based on: |
|---|---|---|
|
Inpatients with therapy-induced hypoproliferative thrombocytopenia (non-immune) |
Transfuse one dose when platelet count is less than |
2025 AABB and ICTMG Clinical Practice Guidelines,13 ASCO15 and BCSH14, Choosing Wisely Canada |
| Central venous catheter placement at anatomic sites amenable to manual compression | Transfuse one dose if the platelet count is less than 10 x 109 /L. |
2025 AABB and ICTMG International Clinical Practice Guidelines |
|
Elective diagnostic lumbar puncture (LP) Low-risk interventional radiology procedures |
Transfuse one dose if the platelet count is less than 20 x 109/L, and check the platelet count before starting procedure. |
2025 AABB and ICTMG International Clinical Practice Guidelines,13 Guidelines of the BCSH14 and the Society of Interventional Radiology17 |
| Therapeutic anticoagulation that cannot be stopped |
Consult thrombosis/hemostasis specialist. Transfuse one dose if the platelet count is less than30 x 109/L |
Expert opinion |
| Patients with acute promyelocytic leukemia (APL) | Transfuse one dose if the platelet count is less than 30–50 x 109/L. |
2019 European LeukemiaNet Recommendations18, 2014 Canadian APL consensus19 |
|
Major elective non-neuraxial surgery High-risk interventional radiology procedures |
Transfuse one dose if the platelet count is less than 50 x 109/L, and check platelet count before starting procedure. |
2025 AABB and ICTMG International Clinical Practice Guidelines,13 Guidelines of the Society of Interventional Radiology17 |
|
Neuraxial surgery or surgery involving the posterior segment of the eye |
Transfuse one dose if the platelet count is less than |
Guidelines of the BCSH14; expert opinion13 |
| Invasive procedure for patients on anti-platelet therapy (e.g., ASA and P2Y12 inhibitors) | Platelet transfusions before an elective procedure should not
be used if anti-platelet agents have not been discontinued. For an urgent invasive procedure, platelet transfusions may be effective but must be tailored to the individual based on the specific level of platelet inhibition and should be done in consultation with transfusion medicine experts. |
Guidelines of the BCSH14; expert opinion |
Notes:
- These clinical practice recommendations were compiled from a review of evidence-based guidelines, Choosing Wisely and Choosing Wisely Canada lists and current literature. Because a formal literature search was not part of the preparation of these recommendations, they are presented as recommendations rather than guidelines.
- Association for Advancement of Blood and Biotherapies (AABB), the International Collaboration for Transfusion Medicine Guidelines (ICTMG), the American Association of Clinical Oncology (ASCO) and the British Committee for Standards in Hematology (BCSH).
- The BCSH guideline recommends prophylactic platelet transfusion for patients with reversible hypoproliferative thrombocytopenia induced by intensive chemotherapy or allogeneic hematopoietic stem cell transplant (HSCT). They suggest considering not giving prophylactic platelet transfusions to well patients with no evidence of bleeding who had an autologous HSCT. The ASCO guideline also suggests that, after autologous HSCT, adult patients should be transfused at the first sign of bleeding rather than prophylactically in experienced centers.
- For some procedures at very low risk of bleeding (e.g., bone marrow aspirate or trephine biopsy, peripherally inserted central catheters (PICCs), traction removal of tunneled CVCs, or cataract surgery), the BCSH guideline recommends considering not giving routinely platelet transfusions before procedure.
The recommendation for transfusing hospitalized adult patients with therapy-induced thrombocytopenia and a platelet count <10 x 109/L is based on several randomized controlled trials (RCTs). The largest RCTs20, 21 compared patients with and without prophylactic platelet transfusion and demonstrated reduced risk of clinically significant (grade 2 or greater) bleeding. Based on subgroup analyses, some populations were identified as being at higher risk for bleeding than others (e.g., allogeneic stem cell transplant recipients having higher risk than autologous stem cell transplant recipients).20-23
For asymptomatic outpatients with irreversible hypoproliferative thrombocytopenia because of chronic marrow failure (e.g., in aplastic anemia or myelodysplastic syndrome) where recovery is not anticipated, a “no prophylactic platelet transfusion’’ strategy is appropriate while not receiving intensive treatment or on low-dose oral chemotherapy.14, 15 Although there is little evidence to inform practice, many such patients have minimal or no significant bleeding for long periods of time despite low platelet counts.
Platelet thresholds: For patients without bleeding and no additional risk factors for bleeding, transfusion of platelets is not indicated unless the platelet count is less than or equal to 10 x 109 per litre. Two large RCTs and one prospective controlled cohort study demonstrated that lowering the prophylactic platelet transfusion threshold from 20 x 109 per litre to 10 x 109 per litre would decrease platelet utilization by more than 20% without increasing major bleeding.24, 25 The evidence to support specific thresholds for platelet transfusions in patients with risk factors for bleeding is limited. For patients at an increased risk of bleeding due to infection or anticoagulant use, higher thresholds (e.g., 15 x 109 per litre) for prophylactic platelet transfusions have been used but evidence supporting this practice is lacking.
Platelet dose: In Canada, platelet components manufactured by Canadian Blood Services have a volume of approximately 264 to 273 mL for untreated (not pathogen-reduced) platelets in PAS and 170 to 281 mL for pathogen-reduced platelets, with a platelet content of approximately 254 to 304 x109 per unit for untreated platelets and 210 to 276 x 109 per unit for pathogen-reduced platelets. These volumes are from the Canadian Blood Services Circular of Information pages for platelets, please refer to these pages for the most up to date volumes and see Chapter 19 for more information on pathogen-reduced platelets.
One bag of platelet concentrate generally raises the platelet count by approximately 15 to 25 x 109 per litre in a 70 kg adult. In practice, the post-transfusion platelet count often does not rise to the expected level. Sepsis, alloimmunization, fever, ITP, or disseminated intravascular coagulation (DIC) may contribute to a suboptimal response. Although currently available data suggest a decreased platelet increment and time interval between transfusions with pathogen-reduced platelets, the differences were small.26
For prophylactic platelet transfusions, higher doses of platelets increase post-transfusion platelet counts and prolong the time until the next transfusion, but recent studies including a large RCT27 suggest that higher doses of platelets do not reduce bleeding and increase total platelet utilization in patients requiring repeated platelet transfusions. A lower dose of platelets (equivalent to half of a standard platelet unit either pooled or apheresis) has been shown to be as clinically effective as one standard dose to prevent bleeding.27 Despite more frequent platelet transfusion events, the lower dose reduces total platelet utilization. Thus, in times of platelet shortages, lower or split platelet doses can be a useful strategy to manage inventory challenges.
Indications in neonatal and pediatric patients
While many indications for platelet transfusion in pediatric populations are similar to those for adults, this population does have unique physiology and should not be considered “small adults”. Transfusion in pediatric patients should be guided by the underlying pathophysiology, bleeding risk, age, weight, and comorbid conditions. When possible, a pediatric transfusion expert should be consulted.
Additional discussion on this topic is also available in Chapter 13 of this Guide.
Platelet transfusions in neonates
Platelet transfusions are provided to neonates who are thrombocytopenic. Thrombocytopenia is the most common hematologic abnormality found in patients admitted to the neonatal intensive care unit.28 In the first 90 days of life, a normal platelet count for a late pre-term infant (more than 33 weeks gestational age) is 123–450 x 109 per litre, while it is 104–450 x 109 per litre for infants born less than 32 weeks gestational age.28
There are generally two indications for platelet transfusions in thrombocytopenic neonates:
- Prevention of a spontaneous or provoked bleed by prophylactically transfusing platelets above a critical threshold.
- For the treatment of active bleeding.
The majority of neonatal platelet transfusions are for prevention. Risk factors for hemorrhage in thrombocytopenic neonates include:
- prematurity
- low and very low birthweight
- twin or multiple pregnancy
- underlying diagnosis of fetal and neonatal alloimmune thrombocytopenia (FNAIT)
- severity of thrombocytopenia
Two previously published clinical trials support a more restrictive platelet transfusion strategy using a threshold of less than 25 x 109 per litre for prophylactic platelet transfusion in non-bleeding neonates. Use of higher thresholds for prophylactic platelet transfusions has failed to demonstrate prevention of intraventricular hemorrhage or other types of bleeding. In fact there is evidence of increased incidence of major bleeding or death in the short term.29, 30 For more information about platelet transfusion administration in neonates is available in Chapter 13 of this Guide.
Table 3. Suggested transfusion thresholds for neonates with non-immune thrombocytopenia†.
| Clinical setting | Platelet count threshold (x109 per litre) |
|---|---|
| No bleeding | Less than 25 |
| Neonate with bleeding or before surgery or invasive procedure | Less than 50 |
| Neonate with major bleeding (e.g., ICH) or requiring neuraxial surgery | Less than 100 |
†These clinical practice recommendations are based on a review of the current literature and published guidelines13, 31, 32 on platelet transfusions in neonates. Because a formal literature search was not part of the preparation of these recommendations, they are presented as recommendations rather than guideline.
Neonates with FNAIT were not included in the above randomized trials.
The International Collaboration for Transfusion Medicine Guidelines (ICTMG) recommendations for postnatal management of FNAIT33 suggest using an initial threshold of 100 x 109 per litre in neonates with FNAIT and intracranial or gastro-intestinal bleeding and then a threshold of 50 x 109 per litre for seven days. ICTMG also recommends using a threshold of 30 x 109 per litre for neonates without evidence of life-threatening bleeding. If available, HPA-compatible platelets should be used; however, if unavailable, HPA unselected platelets should be transfused rather than no transfusion. HPA-compatible platelets may be HPA selected platelets from compatible allogeneic donors or maternal platelets. To prevent transfusion-associated graft vs host disease (TA-GVHD), maternal platelets should be either irradiated or psoralen treated ensuring lack of viable residual lymphocytes. See Chapter 12 of this Guide for more information about the serologic investigations and treatment of subsequent pregnancy management for women who previously delivered an infant with FNAIT.
Platelet transfusions in children
Guidelines for platelet transfusions for children are similar to those for adults (recommended doses for pediatric and neonatal platelet transfusions are provided in the Canadian Blood Services Circular of Information), although children are underrepresented in trials that studied prophylactic treatment for hospitalized patients with thrombocytopenia. The decision to transfuse platelets in children should consider the etiology and expected natural history of the thrombocytopenia. In children with ITP, as in adults, platelet transfusions should be considered only in cases of severe bleeding.
Platelet selection
Platelets have A and B antigens on their cell surface but do not express the Rh antigens. Ideally, ABO-identical platelet transfusions should be transfused, but non-identical ABO transfusions can be given if ABO-matched platelets are not available. Risks associated with incompatible platelet transfusions are shown in Figure 1.
Some evidence suggests that ABO-mismatched platelets are a risk factor for poor platelet count increments.34 However, there is limited clinical significance. A secondary analysis of the PLADO trial demonstrated that although the ABO-identical and fresher platelets had modestly increased post-transfusion platelet increments compared with whole blood, non-identical and/or older platelets, there was no measurable impact on the prevention of clinical bleeding.3, 27
Hemolysis due to naturally occurring anti-A and anti-B antibodies (also called isohemagglutinins) in the plasma of mismatched ABO platelet transfusions have been reported. The concentration of isohemagglutinins is lower in psoralen-treated and PAS-E suspended platelet concentrates. Plasma reduction of platelet units can be considered to decrease the overall exposure to these antibodies. Transfusion laboratories often determine anti-A/anti-B titres of group O platelets (supernatant) and avoid use of group O platelets units in non-group O patients when the titres exceed a pre-determined threshold. Canadian Blood Services determines anti-A/anti-B titres on all whole blood and apheresis donations. If the titres are below the predetermined threshold in all donations contributing to a platelet pool, or in an apheresis platelet donation, the corresponding component will be labeled “Low Anti-A/B.” More information can be found in our FAQ: Donor high titre isohemagglutinin (anti-A/anti-B) testing at Canadian Blood Services.
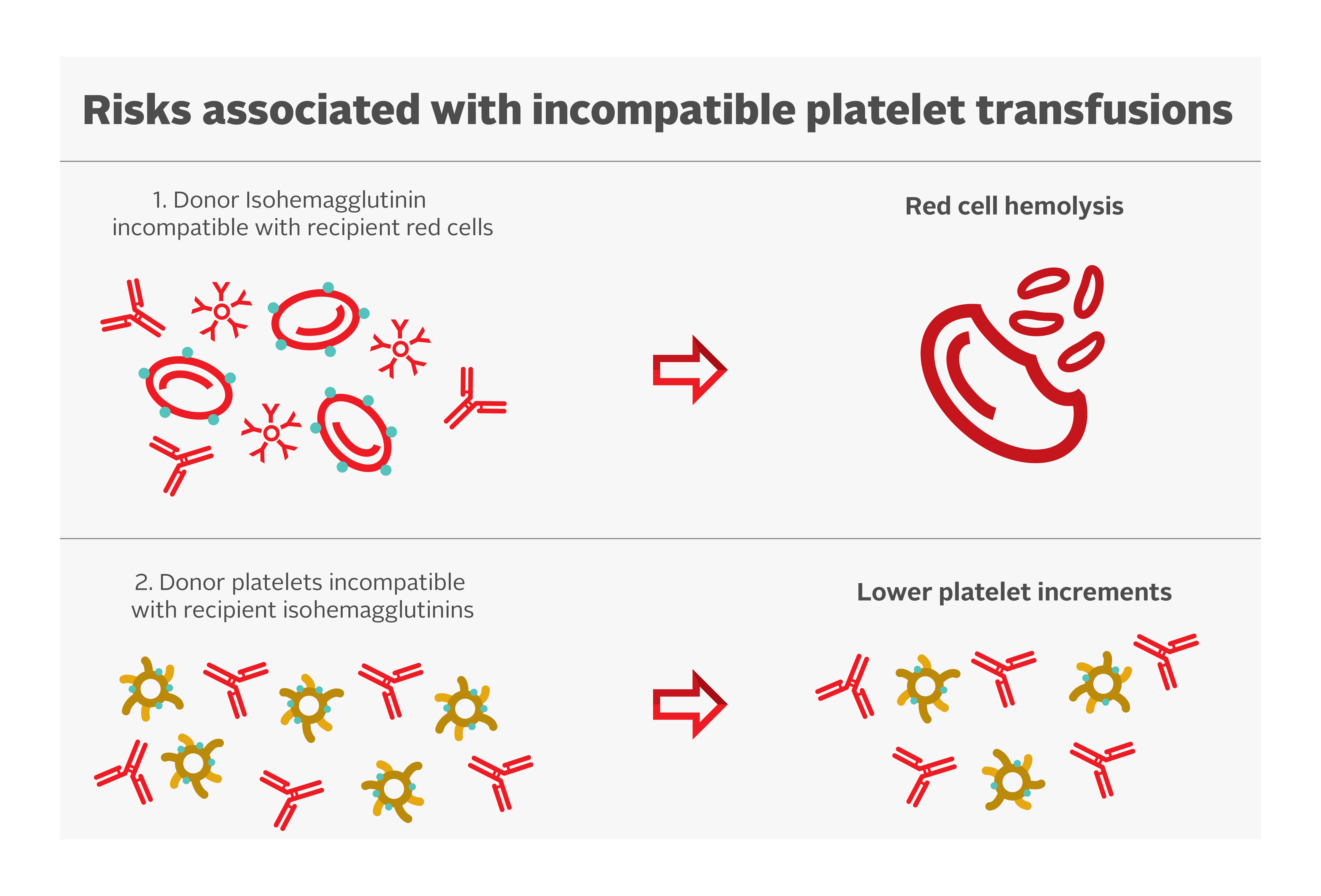
While platelets do not express Rh antigens, platelet components may contain small amounts of red blood cells. As a result, transfusing platelets from Rh positive donors to Rh negative recipients can result in the recipient producing antibodies targeting the RhD antigen, which may interfere with future transfusions or complicate pregnancies. Therefore, administration of anti-D immune globulin (RhIg) may be considered for all RhD-negative children and individuals of reproductive age within 72 hours of platelet transfusion from an RhD-positive donor.35, 36 Evidence suggests a low rate of alloimmunization among those receiving RhD antigen incompatible platelet transfusions without anti-D immune globulin in the general population.37 Clinical factors such as recent chemotherapy or immunosuppression can reduce the rate of alloimmunization and might affect the decision to give anti-D immune globulin. Apheresis platelet units manufactured with current protocols have minimal contamination with red blood cells38 and therefore the rate of alloimmunization after Rh-incompatible apheresis platelet transfusions is extremely low.39, 40
Platelets also express HLA class I antigens and antigens specific to platelets, the HPAs. HLA and/or HPA alloimmunization are a cause of refractoriness to platelet transfusions; see section on platelet refractoriness.
Adverse reactions
Platelet transfusions are associated with both infectious and non-infectious adverse effects (Table 4). Also see Chapter 10 of this Guide and Canadian Blood Services’ summary of adverse transfusion reactions.
Bacterial contamination
While each unit of platelets has the same risk of transmitting viral infections as a unit of red blood cells, bacterial infections are a particular concern with platelets because they are stored at room temperature.
The source of bacterial contamination is usually skin flora acquired during blood collection or less commonly, bacteremia in the donor. A combination of strategies are used to identify and reduce the risk of bacterial contamination of a platelet component. This includes diversion of the first 10 mL of collection to remove the skin plug, routine bacterial screening of all non-pathogen inactivated components, and the introduction of psoralen-treated pathogen inactivated platelet concentrates. For more information on platelet bacterial testing, see the FAQ on Canadian Blood Services platelet bacterial screening and see Chapter 19 of this Guide for more about pathogen-reduced platelets. Annual rates of bacterial contamination are also available in Canadian Blood Services’ annual Surveillance Report.
Alloimmune refractoriness
Platelet transfusions sometimes have the unique complication of alloimmune refractoriness. In this condition, routine platelet transfusions do not increase the recipient’s platelet count because they are quickly destroyed by the recipient’s immune system. This occurs in patients who have anti-HLA antibodies or who develop anti-platelet antibodies after a blood transfusion or a pregnancy. The risk of alloimmune refractoriness is significantly reduced (to approximately four per cent of recipients) by universal leukoreduction of all blood components.41 The risks of alloimmunization and platelet refractoriness are similar with platelets derived from whole blood versus apheresis since both components are leukoreduced. Adequate increments in the post-transfusion platelet counts in these patients can only be achieved by the transfusion of HLA- or HPA-selected platelets (see below, platelet refractoriness).
Transfusion associated graft versus host disease (TA-GvHD)
Although transfusions with apheresis platelets may reduce exposure to different donors, apheresis platelets from an HLA-matched donor or a blood relative may increase the risk of TA-GvHD. To prevent this rare complication, all HLA-matched blood components should be irradiated to destroy residual lymphocytes, unless they are pathogen-reduced platelets (see NAC recommendations for use of irradiated blood components in Canada).
Table 4: Adverse reactions from platelet transfusions.42-46 Source: Adapted from Canadian Blood Services 2024 Circular of Information.47
|
Event |
Approximate frequency |
Symptoms and signs |
Notes |
|---|---|---|---|
|
Mild allergic reaction |
1 in 100 |
Urticaria, pruritus and/or erythema |
Transfusion can be restarted after assessment and necessary intervention |
|
Febrile non-hemolytic transfusion reactions (FNHTR) |
0.5-2 in 100 |
Fever, chills and/or rigor |
Diagnosis of exclusion. A patient with fever should be evaluated for other more serious transfusion reactions. |
|
Transfusion associated circulatory overload (TACO) |
0.1 - 1 in 100 |
Dyspnea, orthopnea, cyanosis, tachycardia, raised venous pressure and/or hypertension. |
Due to excessive volume or excessively rapid transfusion rates. May be difficult to distinguish from TRALI |
|
Septic reaction |
Rare |
Fever, chills, rigors, nausea, vomiting, diarrhea, abdominal and muscle pain, hypotension, hemoglobinemia, and disseminated intravascular coagulation and/or renal failure. |
Approximate frequency per platelet concentrate based on Canadian Blood Services data:
As reported by other international blood agencies:
|
|
Transfusion related acute lung injury (TRALI) |
0.5 - 1 in 100,000 |
New onset of hypoxemia, new bilateral lung infiltrates on chest X-ray and no evidence of circulatory overload. |
Occurs during or within 6 hours of transfusion. May be difficult to distinguish from TACO. |
|
Post transfusion purpura (PTP) |
Very rare |
Abrupt onset of severe thrombocytopenia 1 – 24 days post transfusion. |
Most cases of PTP occur in recipients who are HPA-1b homozygous receiving HPA-1a positive blood components. |
|
Transfusion-related alloimmune thrombocytopenia |
Rare |
Abrupt onset of potentially severe thrombocytopenia within hours of transfusion. |
Passive transfer of platelet antibodies leading to thrombocytopenia. |
|
Immediate hemolytic transfusion reactions (HTR) |
Rare |
Fever, chills, hemoglobinuria, dyspnea, shock, disseminated intravascular coagulation, chest pain and/or back pain. |
May be associated with ABO plasma incompatibility. |
|
Anaphylaxis |
Rare |
Hypotension, upper and/or lower respiratory obstruction, anxiety, nausea and vomiting. |
Resuscitation according to institutional guidelines. IgA-deficient patients who have formed anti-IgA antibodies may experience anaphylactic reactions. However, in most cases of anaphylactic reactions, no specific antibodies are found in the patient. |
|
Graft-versus-host disease (GVHD) |
Very rare |
Pancytopenia, rash, liver dysfunction, diarrhea. |
Irradiated cellular blood components eliminate this risk. |
|
Isolated hypotensive reaction |
Unknown |
Hypotension, occasionally accompanied by urticaria, dyspnea and nausea. |
Diagnosis of exclusion. May occur more frequently in patients on angiotensin-converting enzyme (ACE) inhibitor. |
|
Infectious disease |
See Surveillance Report for residual risk of tested viruses |
Variable according to infectious disease. |
There are other transfusion- transmissible pathogens other than HIV, HBV, HCV, HTLV I/II and WNV as well as parasites and prions. |
Management of special situations
Platelet refractoriness
Platelet refractoriness is a major complication in the management of thrombocytopenic patients. Platelet refractoriness may be due to immune-mediated destruction in approximately 20% of patients and non-immune mediated in 80% of the patients. See Figure 2 for an algorithm for managing platelet refractoriness.
The causes of non-immune refractoriness include fever, infection, drugs, splenomegaly, and disseminated intravascular coagulation. Obtaining detailed patient history and physical exam findings helps to elucidate cause and determine goals and urgency of treatment.
In patients with poor responses to platelet transfusions, measuring the post-transfusion platelet count approximately one hour after the platelet transfusion can help distinguish between immune and non-immune causes. Studies have used various calculations48 to define platelet refractoriness (see Box 1).
| Box 1. Various calculations used to define platelet refractoriness |
|---|
|
Platelet Increment (PI) = P2-P1, where P1 is the pre-transfusion platelet count and P2 is the post-transfusion platelet count. Corrected Count Increment (CCI) = [(PI X BSA) / n] x 100, where BSA represents the body surface area in meters2 and n is the number of platelets transfused. The Percentage Platelet Recovery (PPR) = [(PI x body weight (kg) x 0.075 (L/kg)) / n] x 100. Body weight (kg) x 0.075 (L/kg) is an estimate of blood volume. |
In patients with poor response to platelet transfusion, the platelet response measured between 10 and 60 minutes after completion of a platelet transfusion may be used to determine if alloimmunization is the likely cause of the refractoriness. A corrected count increment below 7.5 x 109 per litre at one-hour post-transfusion or a percentage platelet recovery at one-hour post-transfusion under 30 percent are suggestive of alloimmune refractoriness. A good increment at one hour with a fall below 7.5 x 109 per litre at 24 hours suggests non-immune platelet destruction. Note, some institutions may use a corrected count increment between 5.0 to 7.5 x109 per litre. A lower corrected count increment threshold may be used when a patient has multiple conditions that may account for a lower platelet increment in addition to HLA or HPA antibodies.
Since the platelet yield in a platelet unit is not directly measured, a platelet increment below 5–10 x 109 cells one-hour post-transfusion can be used instead of the correct count increment or percentage platelet recovery.
Testing for HLA- or HPA- antibodies can be done with tests such as the lymphocytotoxic antibody assay and flow cytometry. Canadian Blood Services’ National Platelet Immunology Reference Laboratory provides recipient testing for these antibodies. HLA alloimmunization is more frequent than HPA alloimmunization as the cause of alloimmune refractoriness.
Platelet refractoriness and alloimmunization are not interchangeable. Platelet refractoriness due to alloimmunization is managed differently than non-immune refractoriness (see Figure 2). Once anti-HLA or anti-platelet (anti-HPA) antibodies have been identified, select apheresis platelet components may be useful to achieve adequate post-transfusion platelet count increments. At Canadian Blood Services, HLA and/or HPA selected platelet components can be obtained from typed apheresis platelet donors. As selecting and contacting appropriate donors takes time and donors may not be immediately available, there is often a delay of several days prior to receipt of the initial HLA/HPA selected platelet component.
A systematic review on efficacy of HLA-selected platelet transfusions for patients with hypoproliferative thrombocytopenia showed that HLA-selected platelets did not reduce alloimmunization and refractoriness rates beyond that offered by leukoreduction.16 HLA-selected platelets led to better one-hour post-transfusion count increments and percentage of platelet recovery in refractory patients; however, the effect at 24 hours was inconsistent.16 In addition, there is no benefit for HLA/HPA selected apheresis platelet components in recipients who do not have demonstrable anti-HLA or anti-HPA antibodies. There is a paucity of data to support current HLA-matching practices; multi-centre prospective studies comparing approaches designed to detect differences in important patient outcomes and resource implications are needed.49
In practice, there are commonly used guidelines when deciding if HLA selected platelets are needed for a patient. If HLA antibodies are identified, a calculated panel reactive antibody (cPRA) is commonly used to define when HLA selected platelets should be ordered. A cPRA provides an estimated percentage of the population that is incompatible with the tested patient – for example, a cPRA of 80 per cent predicts that 80 per cent of donor platelets would be incompatible and not provide an appropriate platelet increment after transfusion. Most often, any cPRA below 30 per cent is not eligible for HLA selected platelets, because at least one of the donors in a platelet pool should be compatible with a patient when transfused.
It is difficult to match patients with a cPRA over 95 per cent or patients with a high cPRA needing continuous transfusion support (e.g., cPRA over 80 per cent), finding compatible platelets in a timely manner can be difficult. Permissive mismatched platelets can be selected on a case-by-case basis. Low positive HLA antibodies provide equal platelet increments to fully HLA matched platelets and can increase the compatible donor pool to support the transfusion needs of a patient.50, 51
The International Collaboration for Transfusion Medicine Guidelines (ICTMG) made the following recommendations for management of patients with hypoproliferative thrombocytopenia who are refractory to platelet transfusions, which are summarized in Table 5.35
Table 5. Recommendations for management of patients with hypoproliferative thrombocytopenia who are refractory to platelet transfusions from The International Collaboration for Transfusion Medicine Guidelines.35
| Clinical setting | Recommendatino for platelet transfusion | Level of evidence |
|---|---|---|
| HLA class I antibodies | Should probably receive class I HLA-selected or crossmatch-selected platelet transfusions | Weak |
| HPA antibodies | Should probably receive HPA-selected or crossmatch-selected platelet transfusions | Very weak |
| Non-immune causes (e.g., no HLA class I or HPA antibodies) | Should probably not receive HLA-selected or crossmatch-selected platelet transfusions | Very weak |
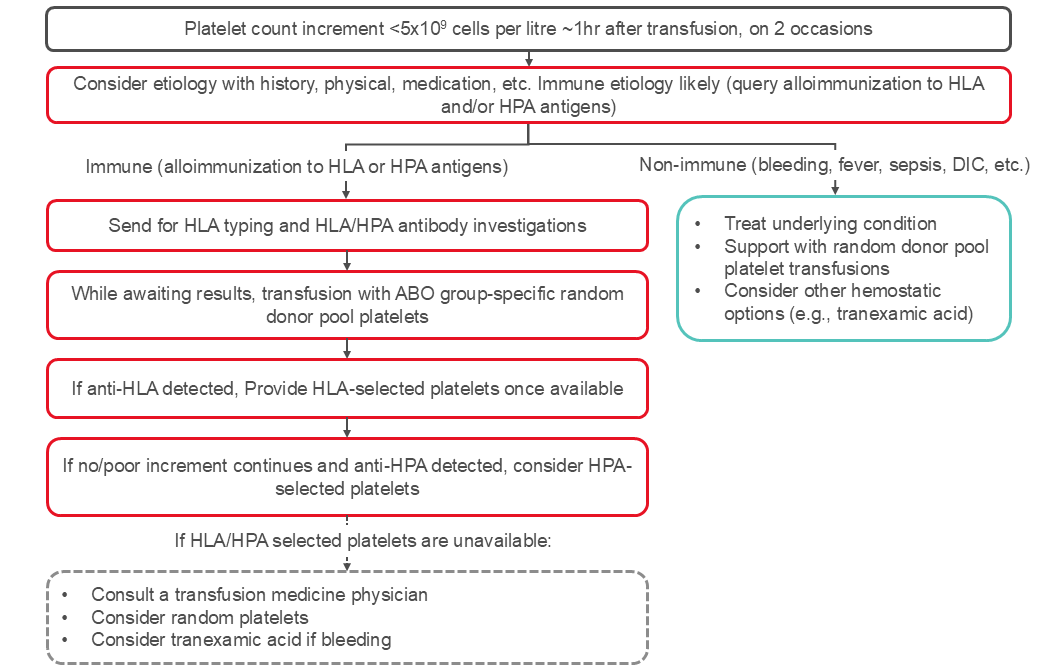
Figure 2: Management of platelet refractoriness. The algorithm reflects published ICTMG guidelines.35
Management of alloimmunized refractory patients who do not respond to HLA- or HPA- selected platelets may be challenging. Despite poor platelet count increments, bleeding patients may still derive hemostatic benefits from platelet transfusions.
Post-transfusion purpura (PTP)
PTP is a rare thrombocytopenic syndrome with an estimated incidence of one or two cases per 100,000 transfusions,52, 53 and may account for 0.04% of all reported transfusion reactions.54 Platelet transfusions are the most common inciting event, but PTP may also rarely occur following red blood cell transfusion. The incidence between 1996–1999 was 10.3 cases per year, and this dropped following implementation of leukoreduction in 1999 to 2.3 cases per year between 2000–2005.52
PTP is an immune-mediated reaction against HPA, most frequently against HPA-1a. PTP occurs most often in patients homozygous for HPA-1b, who lack the common HPA-1a platelet antigen. A sensitizing event such as pregnancy or prior transfusion can cause development of anti-HPA-1a antibodies. These antibodies not only destroy HPA incompatible platelets, but also the patient’s own platelets, leading to severe thrombocytopenia. Autoantibodies directed at recipient platelet antigens and antibodies against other platelet antigens have also been described.55-58
PTP presents as profound thrombocytopenia (platelet count below 10 x109 cells per litre), which classically occurs five to ten days following transfusion. Thrombocytopenia typically resolves within weeks but may persist for longer periods of time. PTP patients are at risk for clinically significant bleeding. Mortality has been estimated to be between 5–20%. The majority of PTP episodes occur in female patients with a history of pregnancy, though cases may occur in male patients as well.
PTP diagnosis can be confirmed by detection of platelet specific antibodies and HPA antigen typing. Ruling out other severe thrombocytopenic conditions such as disseminated intravascular coagulopathy (DIC), immune thrombocytopenic purpura (ITP), heparin-induced thrombocytopenia (HIT), thrombotic thrombocytopenic purpura (TTP) and consumptive thrombocytopenia is critical.
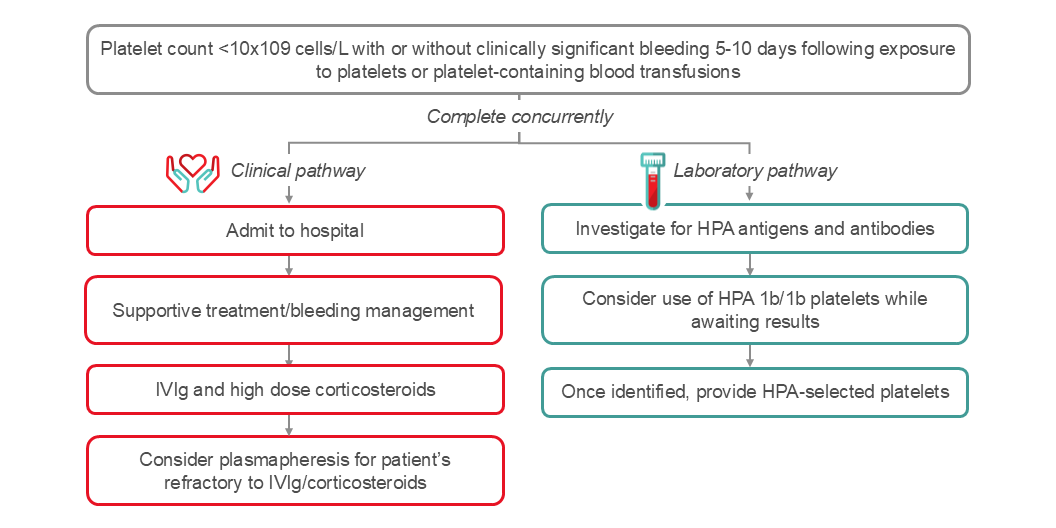
An algorithm for the management of PTP is shown in Figure 3. It is important to rule out other potential causes for severe thrombocytopenia. Goals of treatment are to mitigate risk of bleeding and often require use of emergent multimodal therapy depending on severity of bleeding. Initiation of treatment should be implemented without waiting for the results of serological investigations. Cornerstones of therapy include high dose IVIg, steroids, and if bleeding, transfusion of HPA compatible platelets. Plasma exchange (2B recommendation) is another treatment option in PTP cases.59
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Sciences’ Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Acknowledgements
The authors acknowledge Tanya Petraszko, MD, FRCPC, and Michelle Zeller, MD, FRCPC, MHPE, DRCPSC, as authors of the previous version of this chapter. We would also like to acknowledge Dr. Andrew Shih for reviewing this chapter.
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
Suggested citation
Lafrance, C., Malcolmson, C., Ning, S., Anani W., Yan, M. Platelet transfusion, alloimmunization and management of platelet refractoriness In: Khandelwal A, Brooks K., editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2025 [cited YYYY MM DD]. Chapter 18. Available from: https://professionaleducation.blood.ca
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
References
- Patel, S. R., Hartwig, J. H., & Italiano, J. E., Jr. (2005). The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest, 115(12), 3348-3354. https://doi.org/10.1172/jci26891
- Brass, L. (2010). Understanding and evaluating platelet function. Hematology Am Soc Hematol Educ Program, 2010, 387-396. https://doi.org/10.1182/asheducation-2010.1.387
- Triulzi, D. J., Assmann, S. F., Strauss, R. G., Ness, P. M., Hess, J. R., Kaufman, R. M., Granger, S., & Slichter, S. J. (2012). The impact of platelet transfusion characteristics on posttransfusion platelet increments and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood, 119(23), 5553-5562. https://doi.org/10.1182/blood-2011-11-393165
- Roberts, I., Shakur, H., Coats, T., Hunt, B., Balogun, E., Barnetson, L., Cook, L., Kawahara, T., Perel, P., Prieto-Merino, D., Ramos, M., Cairns, J., & Guerriero, C. (2013). The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess, 17(10), 1-79. https://doi.org/10.3310/hta17100
- WOMAN Trial Collaborators. (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet, 389(10084), 2105-2116. https://doi.org/10.1016/s0140-6736(17)30638-4
- Crash-3 trial collaborators. (2019). Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet (London, England), 394(10210), 1713-1723. https://doi.org/10.1016/S0140-6736(19)32233-0
- Leclerc, S., Nadeau-Fredette, A. C., Elftouh, N., Lafrance, J. P., Pichette, V., & Laurin, L. P. (2020). Use of Desmopressin Prior to Kidney Biopsy in Patients With High Bleeding Risk. Kidney Int Rep, 5(8), 1180-1187. https://doi.org/10.1016/j.ekir.2020.05.006
- Desborough, M. J., Oakland, K. A., Landoni, G., Crivellari, M., Doree, C., Estcourt, L. J., & Stanworth, S. J. (2017). Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost, 15(2), 263-272. https://doi.org/10.1111/jth.13576
- Andersen, L. K., Hvas, A. M., & Hvas, C. L. (2021). Effect of Desmopressin on Platelet Dysfunction During Antiplatelet Therapy: A Systematic Review. Neurocrit Care, 34(3), 1026-1046. https://doi.org/10.1007/s12028-020-01055-6
- Mohinani, A., Patel, S., Tan, V., Kartika, T., Olson, S., DeLoughery, T. G., & Shatzel, J. (2023). Desmopressin as a hemostatic and blood sparing agent in bleeding disorders. Eur J Haematol, 110(5), 470-479. https://doi.org/10.1111/ejh.13930
- Baharoglu, M. I., Cordonnier, C., Al-Shahi Salman, R., de Gans, K., Koopman, M. M., Brand, A., Majoie, C. B., Beenen, L. F., Marquering, H. A., Vermeulen, M., Nederkoorn, P. J., de Haan, R. J., & Roos, Y. B. (2016). Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet, 387(10038), 2605-2613. https://doi.org/10.1016/s0140-6736(16)30392-0
- Kaufman, R. M., Djulbegovic, B., Gernsheimer, T., Kleinman, S., Tinmouth, A. T., Capocelli, K. E., Cipolle, M. D., Cohn, C. S., Fung, M. K., Grossman, B. J., Mintz, P. D., O'Malley, B. A., Sesok-Pizzini, D. A., Shander, A., Stack, G. E., Webert, K. E., Weinstein, R., Welch, B. G., Whitman, G. J., . . . AABB. (2015). Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med, 162(3), 205-213. https://doi.org/10.7326/M14-1589
- Metcalf, R. A., Nahirniak, S., Guyatt, G., Bathla, A., White, S. K., Al-Riyami, A. Z., Jug, R. C., La Rocca, U., Callum, J. L., Cohn, C. S., DeAnda, A., DeSimone, R. A., Dubon, A., Estcourt, L. J., Filipescu, D. C., Fung, M. K., Goel, R., Hess, A. S., Hume, H. A., . . . Stanworth, S. J. (2025). Platelet Transfusion: 2025 AABB and ICTMG International Clinical Practice Guidelines. JAMA. https://doi.org/10.1001/jama.2025.7529
- Estcourt, L. J., Birchall, J., Allard, S., Bassey, S. J., Hersey, P., Kerr, J. P., Mumford, A. D., Stanworth, S. J., & Tinegate, H. (2017). Guidelines for the use of platelet transfusions. Br J Haematol, 176(3), 365-394. https://doi.org/10.1111/bjh.14423
- Schiffer, C. A., Bohlke, K., Delaney, M., Hume, H., Magdalinski, A. J., McCullough, J. J., Omel, J. L., Rainey, J. M., Rebulla, P., Rowley, S. D., Troner, M. B., & Anderson, K. C. (2018). Platelet Transfusion for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol, 36(3), 283-299. https://doi.org/10.1200/jco.2017.76.1734
- Pavenski, K., Rebulla, P., Duquesnoy, R., Saw, C. L., Slichter, S. J., Tanael, S., & Shehata, N. (2013). Efficacy of HLA-matched platelet transfusions for patients with hypoproliferative thrombocytopenia: a systematic review. Transfusion, 53(10), 2230-2242. https://doi.org/10.1111/trf.12175
- Patel, I. J., Rahim, S., Davidson, J. C., Hanks, S. E., Tam, A. L., Walker, T. G., Wilkins, L. R., Sarode, R., & Weinberg, I. (2019). Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol, 30(8), 1168-1184.e1161. https://doi.org/10.1016/j.jvir.2019.04.017
- Hochhaus, A., Baccarani, M., Silver, R. T., Schiffer, C., Apperley, J. F., Cervantes, F., Clark, R. E., Cortes, J. E., Deininger, M. W., Guilhot, F., Hjorth-Hansen, H., Hughes, T. P., Janssen, J., Kantarjian, H. M., Kim, D. W., Larson, R. A., Lipton, J. H., Mahon, F. X., Mayer, J., . . . Hehlmann, R. (2020). European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia, 34(4), 966-984. https://doi.org/10.1038/s41375-020-0776-2
- Seftel, M. D., Barnett, M. J., Couban, S., Leber, B., Storring, J., Assaily, W., Fuerth, B., Christofides, A., & Schuh, A. C. (2014). A Canadian consensus on the management of newly diagnosed and relapsed acute promyelocytic leukemia in adults. Curr Oncol, 21(5), 234-250. https://doi.org/10.3747/co.21.2183
- Stanworth, S. J., Estcourt, L. J., Powter, G., Kahan, B. C., Dyer, C., Choo, L., Bakrania, L., Llewelyn, C., Littlewood, T., Soutar, R., Norfolk, D., Copplestone, A., Smith, N., Kerr, P., Jones, G., Raj, K., Westerman, D. A., Szer, J., Jackson, N., . . . TOPPS Investigators. (2013). A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med, 368(19), 1771-1780. https://doi.org/10.1056/NEJMoa1212772
- Wandt, H., Schaefer-Eckart, K., Wendelin, K., Pilz, B., Wilhelm, M., Thalheimer, M., Mahlknecht, U., Ho, A., Schaich, M., Kramer, M., Kaufmann, M., Leimer, L., Schwerdtfeger, R., Conradi, R., Dolken, G., Klenner, A., Hanel, M., Herbst, R., Junghanss, C., . . . Study Alliance, L. (2012). Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet, 380(9850), 1309-1316. https://doi.org/10.1016/S0140-6736(12)60689-8
- Murphy, S., Litwin, S., Herring, L. M., Koch, P., Remischovsky, J., Donaldson, M. H., Evans, A. E., & Gardner, F. H. (1982). Indications for platelet transfusion in children with acute leukemia. Am J Hematol, 12(4), 347-356. https://doi.org/10.1002/ajh.2830120406
- Stanworth, S. J., Estcourt, L. J., Llewelyn, C. A., Murphy, M. F., Wood, E. M., & Investigators, T. S. (2014). Impact of prophylactic platelet transfusions on bleeding events in patients with hematologic malignancies: a subgroup analysis of a randomized trial. Transfusion, 54(10), 2385-2393. https://doi.org/10.1111/trf.12646
- Rebulla, P., Finazzi, G., Marangoni, F., Avvisati, G., Gugliotta, L., Tognoni, G., Barbui, T., Mandelli, F., & Sirchia, G. (1997). The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. N Engl J Med, 337(26), 1870-1875. https://doi.org/10.1056/NEJM199712253372602
- Heckman, K. D., Weiner, G. J., Davis, C. S., Strauss, R. G., Jones, M. P., & Burns, C. P. (1997). Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. J Clin Oncol, 15(3), 1143-1149. http://www.ncbi.nlm.nih.gov/pubmed/9060557
- Estcourt, L. J., Malouf, R., Hopewell, S., Trivella, M., Doree, C., Stanworth, S. J., & Murphy, M. F. (2017). Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database of Systematic Reviews, 30(7). https://doi.org/10.1002/14651858.CD009072.pub3
- Slichter, S. J., Kaufman, R. M., Assmann, S. F., McCullough, J., Triulzi, D. J., Strauss, R. G., Gernsheimer, T. B., Ness, P. M., Brecher, M. E., Josephson, C. D., Konkle, B. A., Woodson, R. D., Ortel, T. L., Hillyer, C. D., Skerrett, D. L., McCrae, K. R., Sloan, S. R., Uhl, L., George, J. N., . . . Granger, S. (2010). Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med, 362(7), 600-613. https://doi.org/10.1056/NEJMoa0904084
- Fernández, K. S., & de Alarcón, P. (2013). Neonatal Thrombocytopenia. NeoReviews, 14(2), e74-e82. https://doi.org/10.1542/neo.14-2-e74
- Chen, C., Wu, S., Chen, J., Wu, J., Mei, Y., Han, T., Yang, C., Ouyang, X., Wong, M. C. M., & Feng, Z. (2022). Evaluation of the Association of Platelet Count, Mean Platelet Volume, and Platelet Transfusion With Intraventricular Hemorrhage and Death Among Preterm Infants. JAMA Netw Open, 5(10), e2237588. https://doi.org/10.1001/jamanetworkopen.2022.37588
- Curley, A., Stanworth, S. J., Willoughby, K., Fustolo-Gunnink, S. F., Venkatesh, V., Hudson, C., Deary, A., Hodge, R., Hopkins, V., Lopez Santamaria, B., Mora, A., Llewelyn, C., D'Amore, A., Khan, R., Onland, W., Lopriore, E., Fijnvandraat, K., New, H., Clarke, P., & Watts, T. (2019). Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med, 380(3), 242-251. https://doi.org/10.1056/NEJMoa1807320
- New, H. V., Berryman, J., Bolton-Maggs, P. H., Cantwell, C., Chalmers, E. A., Davies, T., Gottstein, R., Kelleher, A., Kumar, S., Morley, S. L., Stanworth, S. J., & British Committee for Standards in, H. (2016). Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol, 175(5), 784-828. https://doi.org/10.1111/bjh.14233
- AABB. (2009). Pediatric Transfusion: A Physician’s Handbook, 3rd edition.
- Lieberman, L., Greinacher, A., Murphy, M. F., Bussel, J., Bakchoul, T., Corke, S., Kjaer, M., Kjeldsen-Kragh, J., Bertrand, G., Oepkes, D., Baker, J. M., Hume, H., Massey, E., Kaplan, C., Arnold, D. M., Baidya, S., Ryan, G., Savoia, H., Landry, D., & Shehata, N. (2019). Fetal and neonatal alloimmune thrombocytopenia: recommendations for evidence-based practice, an international approach. Br J Haematol, 185(3), 549-562. https://doi.org/10.1111/bjh.15813
- Seigeot, A., Desmarets, M., Rumpler, A., Leroux, F., Deconinck, E., Monnet, E., & Bardiaux, L. (2018). Factors related to the outcome of prophylactic platelet transfusions in patients with hematologic malignancies: an observational study. Transfusion, 58(6), 1377-1387. https://doi.org/10.1111/trf.14592
- Nahirniak, S., Slichter, S. J., Tanael, S., Rebulla, P., Pavenski, K., Vassallo, R., Fung, M., Duquesnoy, R., Saw, C. L., Stanworth, S., Tinmouth, A., Hume, H., Ponnampalam, A., Moltzan, C., Berry, B., Shehata, N., & International Collaboration for Transfusion Medicine Guidelines. (2015). Guidance on platelet transfusion for patients with hypoproliferative thrombocytopenia. Transfus Med Rev, 29(1), 3-13. https://doi.org/10.1016/j.tmrv.2014.11.004
- Murphy, M. F., Brozovic, B., Murphy, W., Ouwehand, W., & Waters, A. H. (1992). Guidelines for platelet transfusions. British Committee for Standards in Haematology, Working Party of the Blood Transfusion Task Force. Transfus Med, 2(4), 311-318. http://www.ncbi.nlm.nih.gov/pubmed/1339584
- Cid, J., Lozano, M., Ziman, A., West, K. A., O'Brien, K. L., Murphy, M. F., Wendel, S., Vazquez, A., Ortin, X., Hervig, T. A., Delaney, M., Flegel, W. A., Yazer, M. H., & Biomedical Excellence for Safer Transfusion, c. (2015). Low frequency of anti-D alloimmunization following D+ platelet transfusion: the Anti-D Alloimmunization after D-incompatible Platelet Transfusions (ADAPT) study. Br J Haematol, 168(4), 598-603. https://doi.org/10.1111/bjh.13158
- Lozano, M., & Cid, J. (2003). The clinical implications of platelet transfusions associated with ABO or Rh(D) incompatibility. Transfus Med Rev, 17(1), 57-68. https://doi.org/10.1053/tmrv.2003.50003
- Cid, J., Ortin, X., Elies, E., Castella, D., Panades, M., & Martin-Vega, C. (2002). Absence of anti-D alloimmunization in hematologic patients after D-incompatible platelet transfusions. Transfusion, 42(2), 173-176. http://www.ncbi.nlm.nih.gov/pubmed/11896331
- O'Brien, K. L., Haspel, R. L., & Uhl, L. (2014). Anti-D alloimmunization after D-incompatible platelet transfusions: a 14-year single-institution retrospective review. Transfusion, 54(3), 650-654. https://doi.org/10.1111/trf.12341
- The Trial to Reduce Alloimmunization to Platelets Study Group. (1997). Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. . N Engl J Med, 337(26), 1861-1869. https://doi.org/10.1056/NEJM199712253372601
- Public Health Agency of Canada. Supplement guideline for investigation of suspected transfusion transmitted bacterial contamination. Canada Communicable Disease Report 2008; 34S1:1-8. http://www.phac-aspc.gc.ca/hcai-iamss/index-eng.php
- Yazer, M. H., Podlosky, L., Clarke, G., & Nahirniak, S. M. (2004). The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion, 44(1), 10-15. http://www.ncbi.nlm.nih.gov/pubmed/14692961
- Popovsky, M. A. (2007). Transfusion Reactions (3rd Edition ed.).
- Pavenski, K., Webert, K. E., & Goldman, M. (2008). Consequences of transfusion of platelet antibody: a case report and literature review. Transfusion, 48(9), 1981-1989. https://doi.org/10.1111/j.1537-2995.2008.01796.x
- Callum, J., Lin, Y., Pinkerton, P. H., Karkouti, K., Pendergrast, J. M., Robitaille, N., Tinmouth, A. T., & Webert, K. (2011). Bloody easy 3, blood transfusions, blood alternatives and transfusion reactions: a guide to transfusion medicine. (3rd edition ed.). http://transfusionontario.org/en/cmdownloads/categories/bloody_easy/
- Canadian Blood Services. (2022). Circular of Information, Pooled Platelets LR CPD, Apheresis Platelets,. Retrieved September 12 from https://www.blood.ca/en/hospital-services/products/component-types/circular-information
- Bishop, J. F., Matthews, J. P., Yuen, K., McGrath, K., Wolf, M. M., & Szer, J. (1992). The definition of refractoriness to platelet transfusions. Transfus Med, 2(1), 35-41. http://www.ncbi.nlm.nih.gov/pubmed/1308461
- Stanworth, S. J., Navarrete, C., Estcourt, L., & Marsh, J. (2015). Platelet refractoriness--practical approaches and ongoing dilemmas in patient management. Br J Haematol, 171(3), 297-305. https://doi.org/10.1111/bjh.13597
- Sullivan, J. C., & Peña, J. R. (2022). Use of Human Leukocyte Antigen (HLA)-Incompatible Platelet Units in HLA Platelet-Refractory Patients With Limited Number of or Low-Level HLA Donor-Specific Antibodies Results in Permissive Transfusions. Arch Pathol Lab Med, 146(10), 1243-1251. https://doi.org/10.5858/arpa.2021-0051-OA
- Karafin, M. S., Schumacher, C., Zhang, J., Simpson, P., Johnson, S. T., & Pierce, K. L. (2021). Human leukocyte antigen (HLA)-incompatible mean fluorescence intensity-selected platelet products have corrected count increments similar to HLA antigen-matched platelets. Transfusion, 61(8), 2307-2316. https://doi.org/10.1111/trf.16430
- Williamson, L. M., Stainsby, D., Jones, H., Love, E., Chapman, C. E., Navarrete, C., Lucas, G., Beatty, C., Casbard, A., & Cohen, H. (2007). The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion-associated graft-versus-host disease. Transfusion, 47(8), 1455-1467. https://doi.org/10.1111/j.1537-2995.2007.01281.x
- Menis, M., Forshee, R. A., Anderson, S. A., McKean, S., Gondalia, R., Warnock, R., Johnson, C., Mintz, P. D., Worrall, C. M., Kelman, J. A., & Izurieta, H. S. (2015). Posttransfusion purpura occurrence and potential risk factors among the inpatient US elderly, as recorded in large Medicare databases during 2011 through 2012. Transfusion, 55(2), 284-295. https://doi.org/10.1111/trf.12782
- Politis, C., Wiersum-Osselton, J., Richardson, C., Grouzi, E., Sandid, I., Marano, G., Goto, N., Condeço, J., Boudjedir, K., Asariotou, M., Politi, L., & Land, K. (2022). Adverse reactions following transfusion of blood components, with a focus on some rare reactions: Reports to the International Haemovigilance Network Database (ISTARE) in 2012-2016. Transfus Clin Biol, 29(3), 243-249. https://doi.org/10.1016/j.tracli.2022.03.005
- Woelke, C., Eichler, P., Washington, G., & Flesch, B. K. (2006). Post-transfusion purpura in a patient with HPA-1a and GPIa/IIa antibodies. Transfus Med, 16(1), 69-72. https://doi.org/10.1111/j.1365-3148.2005.00633.x
- Mueller-Eckhardt, C., & Kiefel, V. (1988). High-dose IgG for post-transfusion purpura-revisited. Blut, 57(4), 163-167. http://www.ncbi.nlm.nih.gov/pubmed/3139110
- Taaning, E., & Tonnesen, F. (1999). Pan-reactive platelet antibodies in post-transfusion purpura. Vox Sang, 76(2), 120-123. https://doi.org/31031
- Hawkins, J., Aster, R. H., & Curtis, B. R. (2019). Post-Transfusion Purpura: Current Perspectives. J Blood Med, 10, 405-415. https://doi.org/10.2147/JBM.S189176
- Delaney, M., Wendel, S., Bercovitz, R. S., Cid, J., Cohn, C., Dunbar, N. M., Apelseth, T. O., Popovsky, M., Stanworth, S. J., Tinmouth, A., Van De Watering, L., Waters, J. H., Yazer, M., & Ziman, A. (2016). Transfusion reactions: prevention, diagnosis, and treatment. Lancet, 388(10061), 2825-2836. https://doi.org/10.1016/s0140-6736(15)01313-6