Chapter 12
Hemolytic disease of the fetus and newborn and perinatal immune thrombocytopenia
Background
This chapter reviews the testing and treatments that are relevant for mothers and their fetuses/infants during pregnancy and postnatally to reduce the risks of hemolytic disease of the fetus and newborn (HDFN) and of immune thrombocytopenias.
Hemolytic disease of the fetus and newborn
What is hemolytic disease of the fetus and newborn?
Alloimmune hemolytic disease of the fetus and newborn (HDFN) may occur when a pregnant woman has an antibody against an antigen on the fetal red cells which has been inherited from the father. Many antibodies to red blood cell antigens can cause HDFN, including those from the ABO, Rh and other blood group systems. Women can develop antibodies either through previous pregnancy or transfusion. The maternal antibodies may cross the placenta and bind to the antigen on the fetal red blood cells, triggering their destruction or suppressing erythropoiesis in fetal bone marrow. HDFN presentation ranges from asymptomatic, to jaundice, anemia and, in its worst-case scenario, death (can be life-threatening for the fetus or newborn). The risk for HDFN can be identified by testing the mother with a group and screen during the pregnancy. RhD HDFN can be prevented through passive anti-D administration to suppress the mother’s immune response against the fetal RhD antigen.
Routine serological testing in pregnancy
All pregnant women should undergo serological typing for ABO and RhD antigens and screening tests to detect unexpected red blood cell antibodies (Table 1). These tests should be performed at the initial prenatal visit. The initial antibody screen will determine whether or not women with an RhD antigen negative status (RhD-negative) have been previously exposed to RhD (sensitized) and produced anti-D antibodies. In addition, the screen will identify women with other red blood cell antibodies capable of causing HDFN. RhD-negative women should be tested again for blood group confirmation and for unexpected red blood cell antibodies at 26 to 28 weeks gestation. For those who are RhD-negative this should occur prior to the administration of Rh immune globulin (Rhlg) prophylaxis, but the administration of RhIg should not be withheld pending these results (see Figure 1 and treatment details in later sections of this chapter). All women who have a history of clinically significant red blood cell antibodies or events that may cause antibody formation, such as blood transfusions, pregnancy complications, amniocentesis or chorionic villous sampling, may require additional antibody testing during the second to third trimester regardless of their RhD status. Some guidelines1, 2 recommend repeat ABO Rh and antibody screen on all women at 26 – 28 weeks to confirm ABO and Rh type and to rule out development of antibodies following initial testing (see Figure 1 and Figure 2).
Table 1. Recommended schedule for serological testing in pregnancy.
| Type of pregnancy |
Timing during pregnancy |
Testing |
|---|---|---|
| All pregnancies |
At initial prenatal visit, ideally during the first trimester |
|
| First pregnancy, regardless of Rh type on initial testing |
At 26–28 weeks gestation |
|
| RhD-negative women and RhD-positive women§ |
At 26–28 weeks gestation, before RhIg administration |
|
| Women at risk of having developed red blood cell antibodies* |
28 weeks or thereafter |
|
|
#Antibody identification should be done when antibodies are detected; sample should be collected prior to RhIg injection.
§Canadian Guidelines recommend reassessment of antibody status in all pregnant women at 26–28 weeks regardless of Rh blood group.
*History of clinically significant red blood cell antibodies or of events that may cause antibody formation, such as blood transfusions, pregnancy complications, amniocentesis or chorionic villous are definite indications for repeat testing; some guidelines recommend repeat testing for all women regardless of events which increase risk for alloimmunization.
Adapted from the AABB’s Guidelines for Prenatal and Perinatal Immunohematology.3 |
||
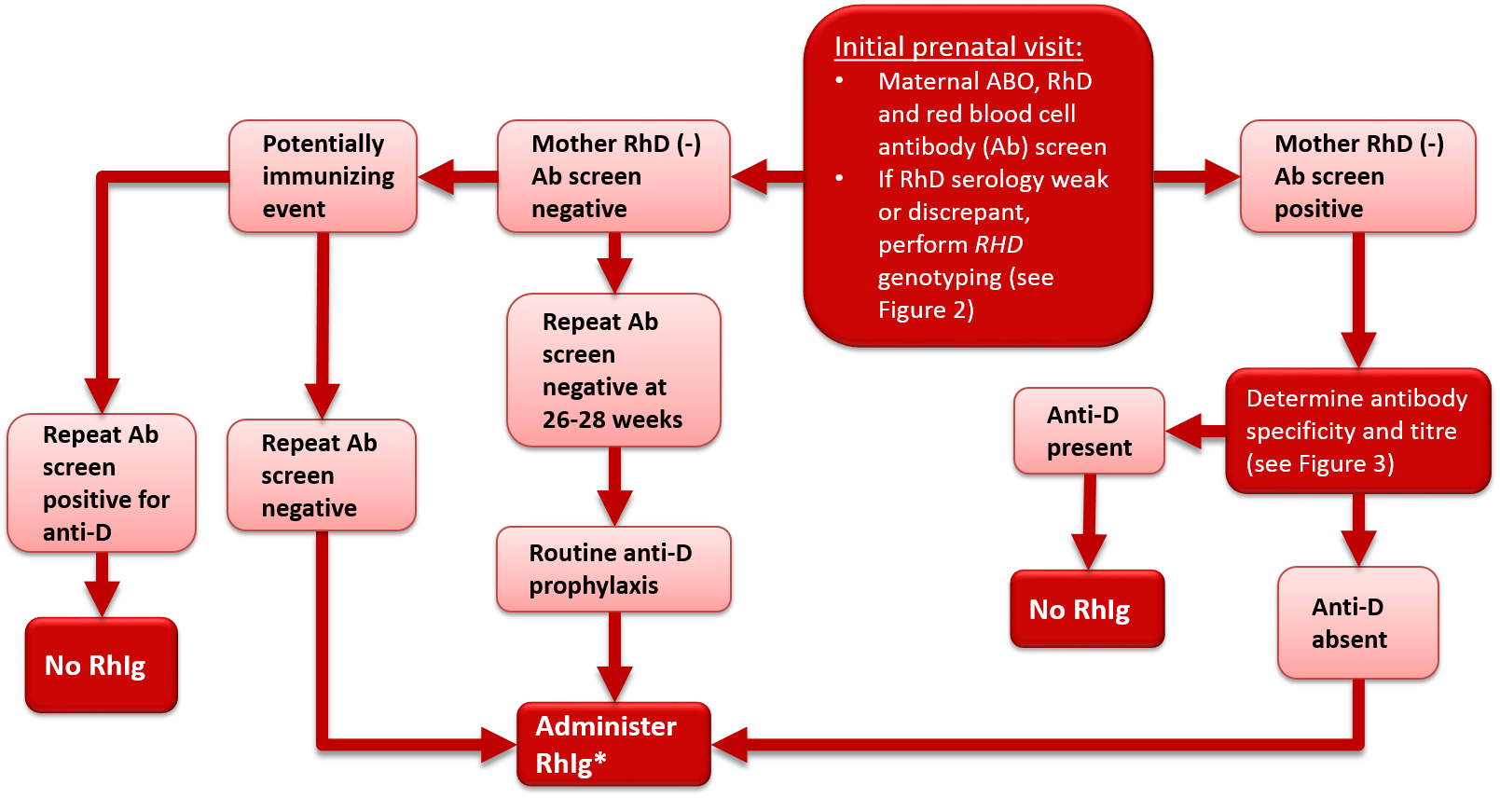
Figure 1. Antenatal serologic testing, RhD-negative mother.
*Administer RhIg if the mother is not known to be immunized to the D antigen and if the fetus is not known to be RhD-negative. Repeat RhIg at 12 week intervals until birth; dosage per package insert. See Table 2 for indications and suggested dosage.
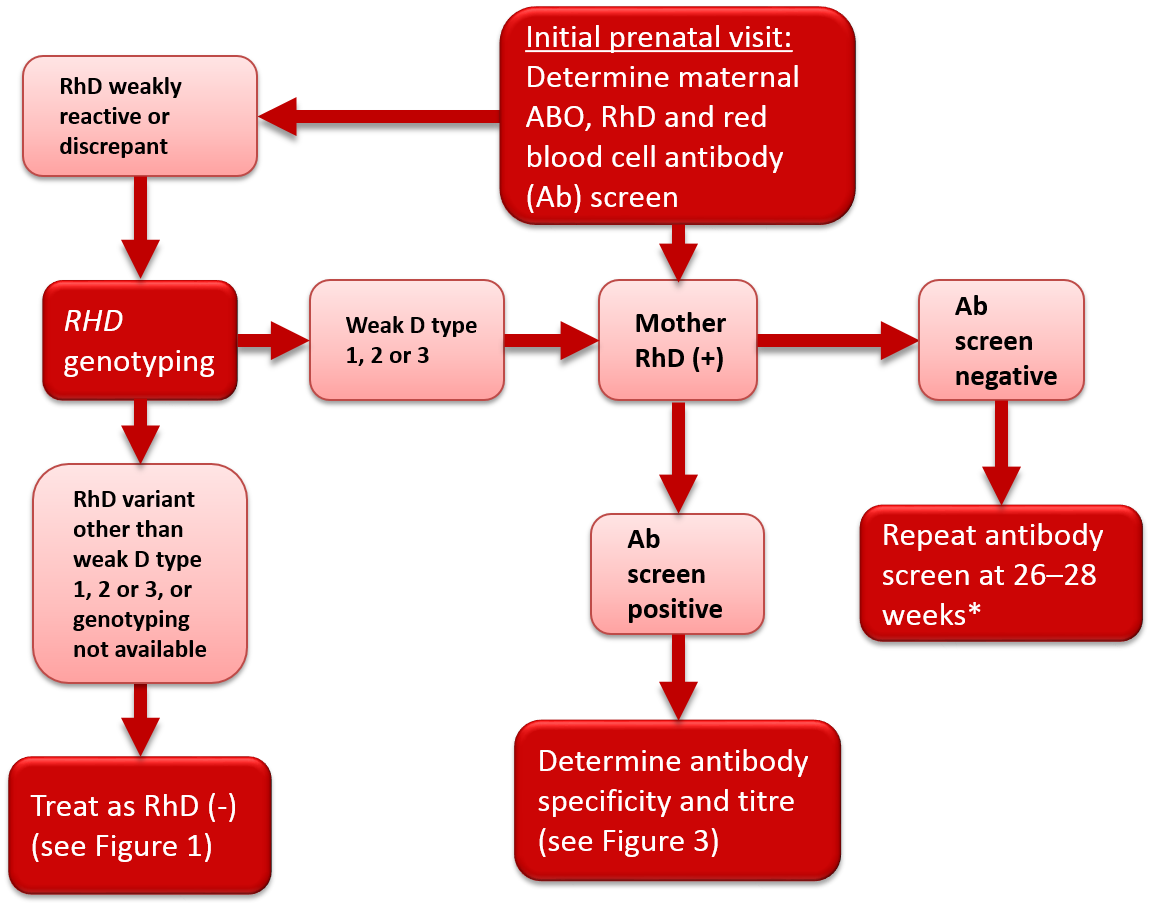
Figure 2. Antenatal serologic testing, RhD-positive mother.
*Policy recommended by the Society of Obstetricians and Gynecologists of Canada (SOGC)2 and the British Committee for Standards in Haematology.1
Identification of RhD variants
Some women may give weak or discrepant results on RhD typing, or current results may differ from historical results. The most common cause of a variant RhD phenotype is a single nucleotide substitution in one RHD allele, although other genetic mechanisms may be involved. A common type of RhD variant is the weak D phenotype. Women with this phenotype have weak expression of the RhD antigen and may present with variable RhD typing depending on the antisera or testing method used in the laboratory. Another type of RhD variant is the partial D phenotype where the RhD antigen is altered, potentially allowing an individual to form alloantibodies to the epitopes on RhD-positive red blood cells that are different than their own. Most weak D and partial D women are at low risk of producing anti-D. Most published guidelines/opinions recommend against weak D serological (IAT phase) testing of women during pregnancy. Similarly, most transfusion practice standards (AABB,4 CSA5 and CSTM6) do not require weak D serological testing for women during pregnancy. In line with these recommendations, Canadian Blood Services’ perinatal testing laboratories do not perform weak D serological testing on RhD-negative pregnant women.2
In 2015, an AABB and American College of Pathologists Joint Working Group provided recommendations for genotyping of pregnant women.7 An algorithm was proposed based on results of manual or automated testing. Individuals with serological results suggesting weak or inconsistent RhD typing were to be referred for genotyping. Individuals found to have weak D type 1, 2 or 3 were considered not at risk for alloimmunization and therefore not candidates for RhIg prophylaxis. As weak D types 1, 2 and 3 comprise the majority of weak D variants in North America, the unnecessary administration of a blood product (RhIg) to a large subgroup of pregnant women would be avoided. The remaining individuals who typed as RhD-negative or carried a different RHD allele were to be considered RhD-negative and at risk of alloimmunization for the purposes of RhIg prophylaxis. A Canadian study of prenatal patients found that 0.4% of women identified by serology as RhD-negative had serological RhD typing variability.8 Of these, 61% were classified as weak D type 1, 2 or 3 following RHD genotyping and could be safely considered RhD-positive and therefore not eligible to receive RhIg prophylaxis.8 If RHD genotyping cannot be performed, it is recommended that women with variable serologic reactivity be considered RhD-negative and eligible for RhIg prophylaxis in order to prevent development of anti-D and reduce the risk of HDFN. The National Advisory Committee on Blood Products (NAC) recommends that “prenatal patients with discrepant, weak or inconclusive serological RhD test results should be further investigated with RHD genotyping to determine RhIg candidacy and optimal Rh type for transfusion."9
In many countries, other than Canada, non-invasive prenatal testing (NIPT) is performed on a blood sample from RhD-negative pregnant women. NIPT allows prediction of the fetal RhD phenotype based on the genotyping of fetal DNA in maternal plasma. RhIg prophylaxis therapy can then be given only to RhD-negative women carrying a RhD-positive fetus. This targeted approach has been shown to prevent the administration of RhIg to about one-third of RhD-negative women due to the fact that they are carrying a RhD-negative fetus.10
Rh immune globulin prophylaxis for the management of non-sensitized RhD-negative women
RhD-negative women with no detectable levels of anti-D (non-sensitized) are treated with RhIg perinatally to prevent alloimmunization and reduce the risk of HDFN. Note that in patients with previous RhIg treatment, passive anti-D persists for many weeks, and this should be considered before withholding routine RhIg prophylaxis. Recommended doses of RhIg are shown in Table 2. RhIg is a plasma protein product that consists primarily of IgG anti-D. RhIg is prepared from pools of human plasma that contain high titers of anti-D. Please refer to Chapter 4 of this Guide for more information about RhIg indications, contraindications, administration and storage.
The mechanism of action of RhIg has not been clearly elucidated; however, its benefits are well documented.11, 12 When RhIg is administered within 72 hours of a full-term delivery of a RhD-positive infant by a RhD-negative mother, the incidence of alloimmunization is decreased from 12–13% to 1–2%. When RhIg is administered at 28 weeks gestation in addition to the postnatal dose, the incidence of alloimmunization is further decreased to 0.1%. One 1,500 IU (300 µg) dose of RhIg protects against immunization by 15 ml of RhD-positive red blood cells (or 30 ml of whole blood).
Where paternity is certain, RhD blood grouping of the baby's father may be offered. If paternity is assured and the baby’s father proves to be RhD-negative, the physician, in consultation with the patient, may choose to withhold RhIg administration to avoid unnecessary blood product exposure. In some circumstances, RHD genotyping of the fetus may be possible from maternal blood samples or from amniocytes or chorionic villous sampling. If the fetus is found to be RhD-negative, RhIg administration may be withheld.
Table 2. Recommended doses of RhIg for RhD-negative women without anti-D during pregnancy.+ ∞
|
Indication |
Dose of RhIg@ |
|---|---|
|
Pregnancy (at 28 weeks gestation)#$ |
300 µg (1,500 IU) IV or IM |
|
Postpartum, if newborn is RhD-positive, including weak D-positive* (within 72 hours of birth) |
120 µg (600 IU) IV or IM or 300 µg (1,500 IU) IV or IM |
|
Threatened abortion§ |
300 µg (1,500 IU) IV or IM |
|
Abortion (including very early pregnancy loss), amniocentesis or chorionic villus sampling after 12 weeks gestation# |
300 µg (1,500 IU) IV or IM |
|
Abortion, amniocentesis, or any other manipulation after < 12 weeks gestation# |
120 µg (600 IU) IV or IM |
|
Other indications†* |
300 µg (1,500 IU) IV or IM |
|
+Where paternity is certain, RhD blood typing of the baby's father may be offered to an RhD-negative woman. If the father is RhD-negative, RhIg prophylaxis may be withheld.
∞If the fetus/newborn is known to be RhD-negative, excluding weak D-positive, RhIg prophylaxis is not required.
#Consideration should be given to repeating RhIg prior to birth if the period without exposure to RhIg prophylaxis exceeds 12 weeks and/or if the RhIg dose provided is < 1,500 IU.
$Alternatively, RhIg 120 µg (600 IU) may be given both at 28 weeks and at 34 weeks gestation.
*Testing must be performed to quantitate fetomaternal hemorrhage. Additional Rhlg will be required if fetomaternal transplacental hemorrhage is determined to be greater than 12 ml of fetal blood (6 ml of fetal red blood cells) for the 600 IU dose or 30 ml of fetal blood (15 ml of fetal red blood cells) for the 1,500 IU dose.
§RhIg may not be necessary with threatened abortion if the fetus is viable and the bleeding stops before 12 weeks gestation. RhIg is recommended following ectopic or molar pregnancy, or with any therapeutic termination of pregnancy regardless of gestational age.
†Other indications include any incident that might result in fetal cells entering the maternal circulation at any time during the pregnancy. These conditions include but are not limited to versions, abdominal trauma, ectopic pregnancy and stillbirth, as well as intraoperative cell salvage used during Caesarean section where the fetal RhD type is unknown.
@RhIg dosing may vary according to local policy and package insert. Note that not all formulations are suitable for IV administration. WinRho ™ distributed by Canadian Blood Services in Canada is suitable for IV or IM administration.
Table adapted from the Society of Obstetricians and Gynaecologists of Canada 2018 recommendations,2 product package insert13 and Qureshi et al.14 |
|
RhIg 300 µg (1,500 IU) should be given routinely to all RhD-negative non-sensitized women at 28 weeks gestation, as well as to women who have an RhD variant other than weak D type 1, 2 or 3 identified on genotyping, and those women with discrepant serological test results on whom genotyping is not possible. RhIg should also be given to all RhD-negative women without antibodies after any incident that might result in fetal cells entering the maternal circulation. These conditions include, but are not limited to, abortion, threatened abortion, amniocentesis, chorionic villus sampling, versions, abdominal trauma, ectopic pregnancy, molar pregnancy and fetal death in utero. The use of intraoperative cell salvage during Caesarean section of a RhD-negative woman without antibodies should also lead to RhIg prophylaxis. If the antepartum dose of RhIg is given prior to 28 weeks gestation, then consideration should be given to repeating RhIg prior to birth if the period without exposure to RhIg prophylaxis exceeds 12 weeks.
Postpartum, within 72 hours of the delivery of a RhD-positive infant, all RhD-negative mothers should receive RhIg 120 µg (600 IU) or 300 µg (1,500 IU). Women with an RhD variant other than weak D type 1, 2 or 3, and those with discrepant serological test results for whom genotyping results are unavailable should also receive RhIg prophylaxis. Dosing may differ in different clinical circumstances and can be determined by consulting the package insert for the specific product, along with local policies. Using the lower dose increases the likelihood of requiring additional RhIg doses following fetal-maternal hemorrhage assessment. A routine test for fetal-maternal hemorrhage is required on all RhD-negative women who deliver a RhD-positive or RhD-unknown fetus (CSA5 and CSTM6 standards). If a semi-quantitative (rosette) screening test is positive, a Kleihauer Betke test or flow cytometry analysis is performed to quantitate the volume of the hemorrhage and to assess the required dose of RhIg to be given (CSA5 and CSTM6 standards). If the quantity of hemorrhage exceeds the capacity of the initial RhIg treatment to provide protection, additional RhIg should be administered. This is particularly important if the lower dose 120 µg (600 IU) of RhIg is administered, and in all cases should be accompanied by a recommendation as to whether additional dose(s) are required. If RhIg is not given within 72 hours of delivery, it should still be given as soon as the need is recognized, up to 28 days after delivery.2
Management of women with red blood cell antibodies
Antibody identification
All women (whether RhD-negative or RhD-positive) with a positive red blood cell antibody screen should have the specificity of the antibody determined at the time of the positive screening test (see Figure 3). If the antibody is a potential cause of HDFN (Table 3), the father should be tested to determine if he expresses the corresponding antigen(s). If paternity is assured and the father is antigen negative, the fetus is not considered at risk. If the father expresses the corresponding antigen(s) then there is a risk that the fetus also expresses these antigen(s) and thus a risk of HDFN. Whenever there is a risk of HDFN, an experienced obstetrician should be consulted early in the pregnancy regarding the treatment plan, and the mother should be referred for investigation and management by an appropriate consultant. If transfusion of either mother or fetus/neonate is a possibility, a transfusion medicine physician should be consulted as early as possible in the pregnancy. Consideration should be given to delivering the infant where neonatal intensive care facilities are available.
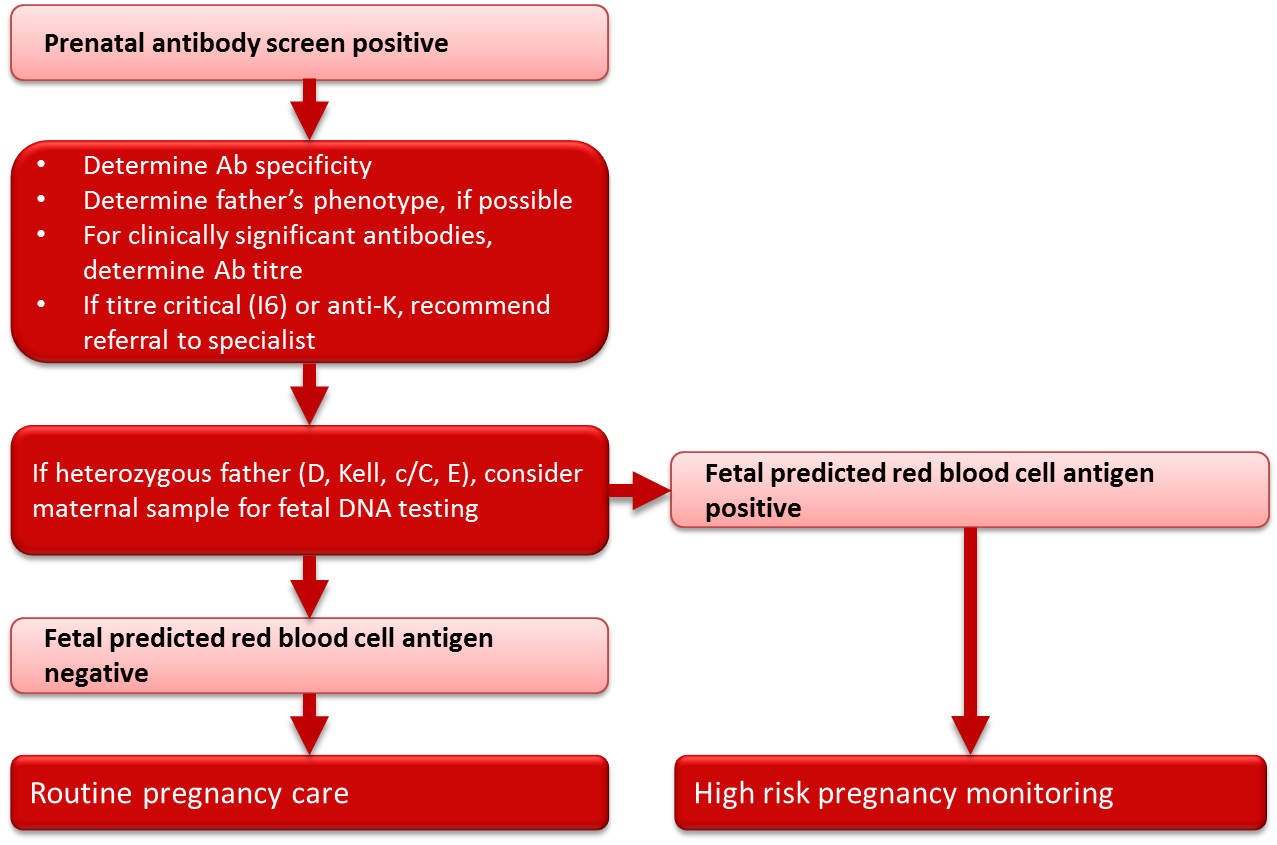
Figure 3. Management of clinically significant red blood cell antibodies in pregnancy.
Table 3. Common red blood cell antibodies that may be associated with HDFN.*
| Indication | System | Antigens |
|---|---|---|
| Associated with severe HDFN |
Rhesus |
D, C, c, E |
| Kell | K, k | |
| Duffy | Fya | |
| Kidd | Jka, Jkb | |
| MNS | M§, S | |
| Associated with mild HDFN |
ABO |
A, B
|
| Ii | i | |
| Duffy | Fyb | |
| Lutheran | Lua, Lub | |
| Not associated with HDFN |
Lewis |
Lea, Leb
|
| Ii | I | |
| P | P1 | |
|
*This list includes only the most common red blood cell antibodies; it is not exhaustive. For less common antibodies refer to Issit and Anstee.15
§Anti-M is clinically significant only when IgG antibody is present. Anti-M may cause late onset neonatal anemia. For more information on anti-M, please see the Anti-M article in the Best Practices section of Canadian Blood Services’ Professional Education website. |
||
Fetal blood group genotyping
In pregnancies complicated by an antibody or antibodies known to cause HDFN (Table 3) and where the father is heterozygous for the particular red blood cell antigen(s), or the father’s antigen status is unknown, genotyping of the fetus may be helpful in determining whether HDFN is likely to occur. With current techniques, genotyping of fetal DNA can be performed on a maternal blood sample with a high degree of accuracy. Genotyping for Rh antigens D, E, C and c, as well as Kell antigens, is available through specialized reference laboratories. Canadian Blood Services and a number of hospital-based prenatal testing programs in Canada currently have programs in place to facilitate the genotyping of fetuses from maternal blood samples for women with critical titres (titre 16) of antibodies to the Rh and Kell antigens. The phenotype of the fetus can be predicted from the genotyping results, and if the results predict that the fetus is negative for the antigen in question, routine pregnancy follow-up can be initiated at the discretion of the treating physician, in place of the high-risk obstetrical care required in the event that the fetus is predicted to be antigen positive. In rare circumstances, for some antigens in addition to Rh and Kell, testing may be performed on DNA extracted from amniocytes following an amniocentesis procedure, or on chorionic villous DNA following chorionic villous sampling. Again, these tests must be outsourced to specialized laboratories and testing is performed only when there is a prior history of HDFN and where knowledge of fetal antigen type would substantially modify pregnancy care. Amniocentesis and chorionic villous sampling are more invasive than the genotyping performed on a maternal blood sample and carry a small, yet significant, risk to the fetus.
Role of transfusion practice in the prevention of HDFN
Transfusing female patients of child-bearing potential carries the risk of red blood cell alloimmunization that could lead to HDFN. The most important way to avoid this risk, and other complications, is to administer transfusions only when absolutely necessary. Some international guidelines16 recommend routine use of Kell-negative red blood cells for transfusion to females of childbearing potential. This strategy for prevention of anti-Kell alloimmunization has been adopted by a number of hospitals and health regions in Canada, and has been shown to be effective in reducing rates of anti-Kell alloimmunization in jurisdictions where it has been in place for a prolonged interval.16, 17
Perinatal thrombocytopenia
Neonatal thrombocytopenia may be caused by decreased production of platelets, increased consumption of platelets, or hemodilution. Preanalytical, spurious causes of thrombocytopenia such as clotted samples following difficult blood collection procedures in neonates must also be considered. Increased consumption of platelets is the most common etiology and may result from a variety of causes including sepsis, medication related effects, necrotizing enterocolitis, disseminated intravascular coagulation, placental insufficiency, congenital infection, asphyxia, or may be immune-mediated.
In general, immune-mediated neonatal thrombocytopenia may be classified into two categories: (1) Fetal and neonatal alloimmune thrombocytopenia (FNAIT), and (2) thrombocytopenia secondary to maternal immune thrombocytopenic purpura (lTP). Maternal ITP is a very rare cause of thrombocytopenia in neonates.
Fetal and neonatal alloimmune thrombocytopenia (FNAIT)
FNAIT may occur when fetal platelets express a paternal antigen that is not found on maternal platelets. Fetal platelets may enter the maternal circulation during gestation or delivery. If the mother becomes alloimmunized, the maternal IgG alloantibody may cross the placenta and cause thrombocytopenia in the fetus. The thrombocytopenia is self-limiting and generally resolves within two to three weeks after delivery. The severity of FNAIT is variable, ranging from mild thrombocytopenia to severe thrombocytopenia with hemorrhage, sometimes occurring in utero.
Serologic investigations: Both parents of an infant with suspected FNAIT should have testing performed, including genotyping for Human Platelet Antigen (HPA) genes that have been associated with FNAIT. In addition, maternal screening for specific anti-platelet antibodies is recommended for diagnosis of this condition along with genotyping of an infant blood sample for the particular HPA genes, if possible. Antibodies to the most common HPA, HPA-1a, cause approximately 90% of FNAIT cases in the Caucasian population. In these cases, the mother is typically HPA-1b homozygous and develops anti-HPA-1a antibodies which cross the placenta and bind to HPA1a antigens on fetal platelets. Anti-HPA-5b and anti-HPA-3a antibodies may also cause this condition, among other antibodies.
Treatment of an affected infant: An infant with FNAIT who requires platelet transfusions should receive HPA-negative platelets, if available. Canadian Blood Services maintains a registry of apheresis platelet donors with particular HPA genotypes, and allogeneic platelets with a particular HPA type can be requested. Any allogeneic donor platelet pools may be used as initial therapy until HPA-negative platelets are available, and are often effective in raising the platelet count even in the presence of anti-HPA antibodies.18 In rare cases, maternal platelets may be used as a source of HPA-negative platelets. As the biological mother's HLA will partially determine fetal haplotype, irradiation of the platelets prior to transfusion is critically important to prevent transfusion-associated graft versus host disease (TA-GvHD) (see Chapter 10, Transfusion reactions for more on TA-GvHD). Although maternal platelets will be negative for the relevant antigen, they are rarely used due to the logistical barriers to collection and processing platelets from a peripartum mother. Intravenous immunoglobulin (IVIg) infusion (1 g/kg/d for up to two days) may be effective and can be considered if platelet transfusions fail to raise the platelet count or if platelets are unavailable. See Chapter 4 of this Guide for more information on the use of immune globulin products.
Treatment of subsequent pregnancies: During pregnancy, women who have previously delivered infants with FNAIT should be followed by a physician experienced in the care of such patients. Guidelines for evidence-based practice for FNAIT are being developed by the International Collaboration for Transfusion Medicine Guidelines.19 A systematic review led by the same group suggests that first-line antenatal management in FNAIT is weekly IVIg administration (1 g/kg), with or without the addition of corticosteroids.20, 21 Apheresis collection of platelets from a donor known to lack the HPA involved may be requested from Canadian Blood Services in advance of delivery.
Maternal immune thrombocytopenic purpura (ITP)
Infants born to mothers with ITP may also have thrombocytopenia due to passive transfer of the maternal autoantibody across the placenta. Most of these infants have only mild thrombocytopenia and the risk of hemorrhage is very low, with the occurrence of intracranial hemorrhage being exceedingly rare.
Infants with thrombocytopenia secondary to maternal lTP rarely require treatment. If platelet transfusions are required, blood group matched allogeneic platelet products are recommended. Other therapies that have been used in severe cases include IVIg and corticosteroids. See Chapter 4 of this Guide for more information on immune globulin products.
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development (CPD) activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Science’s Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Acknowledgements
The authors, Judith Hannon and Gwen Clarke, acknowledge Kathryn Webert, MD, FRCPC, and Heather Hume, MD, FRCPC, as authors of a previous version of this chapter, and thank Lani Lieberman, MD, FRCPC, for reviewing the current version.
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
References
- White J, Qureshi H, Massey E, Needs M, Byrne G, Daniels G, Allard S. Guideline for blood grouping and red cell antibody testing in pregnancy. Transfus Med 2016; 26: 246-63.
- Fung KFK, Eason E. No. 133-Prevention of Rh Alloimmunization. Journal of Obstetrics and Gynaecology Canada 2018; 40: e1-e10.
- Judd WJ, for the Scientific Section Coordinating Committee. Guidelines for Prenatal and Perinatal Immunohematology. Published by AABB, 2005.
- Standards Program Committee (SPC), Blood Banks/Transfusion Services Standards Program Unit (BBTS SPU). Chapter 5: Process Control; Section 5.30: Transfusion-Service-Related Activities: Rh Immune Globulin. In Standards for Blood banks and Transfusion services, 29th edition. Published by AABB, 2014.
- CSA Group. CAN/CSA-Z902-15 Blood and Blood Components. Published in Canada by CSA, 2015.
- CSTM Standards Committee. Standards for Hospital Transfusion Services, Version 4. Published in Markham, Canada by Canadian Society for Transfusion Medicine, 2017.
- Sandler SG, Flegel WA, Westhoff CM, Denomme GA, Delaney M, Keller MA, Johnson ST, Katz L, Queenan JT, Vassallo RR, Simon CD. It's time to phase in RHD genotyping for patients with a serologic weak D phenotype. College of American Pathologists Transfusion Medicine Resource Committee Work Group. Transfusion 2015; 55: 680-9.
- Clarke G, Hannon J, Berardi P, Barr G, Cote J, Fallis R, Alport T, Lane D, Petraszko T, Ochoa G, Goldman M. Resolving variable maternal D typing using serology and genotyping in selected prenatal patients. Transfusion 2016; 56: 2980-5.
- National Advisory Committee on Blood and Blood Products. RHD Genotyping for Prenatal Patients with a Serologically Weak D Phenotype. 2017.
- van der Schoot CE, de Haas M, Clausen FB. Genotyping to prevent Rh disease: has the time come? Curr Opin Hematol 2017; 24: 544-50.
- Bowman J. Thirty-five years of Rh prophylaxis. Transfusion 2003; 43: 1661-6.
- Bowman J. Rh-immunoglobulin: Rh prophylaxis. Best Pract Res Clin Haematol 2006; 19: 27-34.
- Win-Rho SDF [Rho(D) Immune Globulin (Human) for Injection] package insert. Published in Winnipeg, Canada by Cangene Corporation, August 2001.
- Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, Jones J, Allard S. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med 2014; 24: 8-20.
- Issitt PD, Anstee DJ. Applied Blood Group Serology. Published in Durham (N.C.) by Montgomery Scientific Publications, 1998.
- Solheim BG. Provision of K- (KEL1-) blood to women not more than 50 years of age. Transfusion 2015; 55: 468-9.
- Kamphuis MM, Lindenburg I, van Kamp IL, Meerman RH, Kanhai HH, Oepkes D. Implementation of routine screening for Kell antibodies: does it improve perinatal survival? Transfusion 2008; 48: 953-7.
- Bakchoul T, Bassler D, Heckmann M, Thiele T, Kiefel V, Gross I, Arnold DM, DiTomasso J, Smith JW, Paes B, Greinacher A. Management of infants born with severe neonatal alloimmune thrombocytopenia: the role of platelet transfusions and intravenous immunoglobulin. Transfusion 2014; 54: 640-5.
- International Collaboration for Transfusion Medicine Guidelines. Clinical Guidelines and Systematic Reviews. https://www.ictmg.org (Last accessed 2018/04/24).
- Winkelhorst D, Murphy MF, Greinacher A, Shehata N, Bakchoul T, Massey E, Baker J, Lieberman L, Tanael S, Hume H, Arnold DM, Baidya S, Bertrand G, Bussel J, Kjaer M, Kaplan C, Kjeldsen-Kragh J, Oepkes D, Ryan G. Antenatal management in fetal and neonatal alloimmune thrombocytopenia: a systematic review. Blood 2017; 129: 1538-47.
- Bertrand G, Kaplan C. How do we treat fetal and neonatal alloimmune thrombocytopenia? Transfusion 2014; 54: 1698-703.