Lewis system: anti-Lewis
Authors: Sophia Peng, MD and Danielle Meunier, MD
Publication date: October 2019
Primary target audiences: Medical laboratory technologists (MLT) in a hospital laboratory, transfusion medicine physicians
Key points
-
Anti-Lea, anti-Leb, and anti-Leab are not clinically significant.
-
Patients with anti-Lea, anti-Leb, and anti-Leab should receive red blood cell units crossmatch compatible by IAT at 37ºC for transfusion.
-
Patients with sickle cell disease who have anti-Lea, anti-Leb, or anti-Leab should be provided with antigen-negative units for transfusion.
Background
Anti-Le, commonly anti-Lea, Leb, or Leab, are antibodies directed to antigens of the Lewis blood group system. The Lewis antigens are glycoproteins that are found on the surface of many cells and secreted in various body fluids. As such, Lewis, along with ABO and H are sometimes referred to as “histo-blood groups,” given the fact they are present on many different tissue types. Red blood cells acquire these antigens on their membranes by adsorption from circulating antigens. Similar to the modification of the ABO system, the Lewis precursor oligosaccharide, is readily modified by an autosomal dominant-coded fucosyltransferase enzyme (FUT3) as shown in the diagram below. Depending on the combination of Lewis (FUT3) and secretor gene (FUT2), three main phenotypes are possible; Le(a), Le(b), Le(a-b-). The phenotype Le(a+b+) is also possible but is generally only found in people of East Asian descent who possess a weak secretor phenotype.
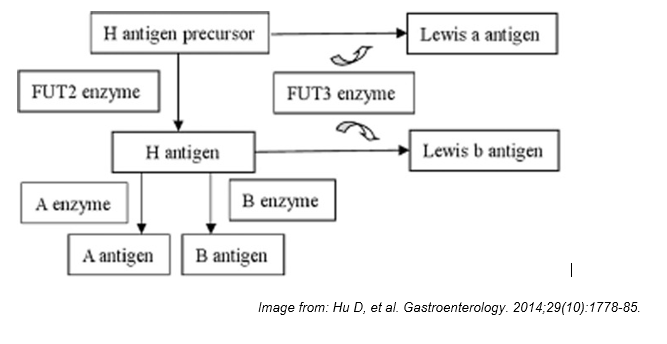
Figure 1: Simplified diagram of how the Lewis system is related to the H and ABO. In non-secretors (no FUT2 enzyme) Lea is formed by addition of a fucose residue to the H precursor. In “secretors” (active FUT2 enzyme) the H antigen in secretions is modified by the FUT3 enzyme to Leb. Le(a-b-) lack an active FUT3 enzyme and can be secretors or non-secretors depending on FUT2, but is not phenotypically obvious.
Anti-Lea, Leb, or Leab are fairly common antibodies. They are usually naturally occurring (i.e. arising without stimulus by transfusion or pregnancy related red blood cell exposure) but can also be immune stimulated. In either case, they are predominantly IgM with some associated IgG component, most commonly found in people with Lewis negative (Le(a-b-)) phenotype. Very rarely, anti-Lea can be found as purely IgG.
Patient management: pre-transfusion and prenatal testing
In the context of transfusion, anti-Lea, Leb, or Leab are almost always clinically insignificant. Only in rare case reports, and mostly with anti-Lea, has it been associated in hemolytic transfusion reactions. The reasoning is three-fold: first, due to IgM predominance, the antibodies are generally not active at body temperature; second, as it is also a highly secreted antigen, the donor plasma antigens neutralize recipient antibodies; and lastly, transfused cells easily shed their antigen and eventually donor membrane antigen matches recipient phenotype.
Similarly, anti-Lea, Leb, or Leab are not generally associated with hemolytic disease of the fetus and newborn (HDFN). Lewis antibodies are commonly detected in prenatal sera because women tend to lose their Lewis antigens during pregnancy, resulting in a temporary Le(a-b-) phenotype and the ability to transiently make anti-Lea, Leb, or Leab until their true phenotype returns, at about 6 weeks postpartum. However, these antibodies are predominantly IgM and do not readily cross the placenta. In addition, although Lewis antigens can be detected in the serum of neonates, they are not expressed on fetal or neonatal red blood cells.
Lewis antibodies are more likely to be IgM and as such will usually react best at room temperature (immediate spin phase). When anti-Lea, Leb, or Leab are detected in a patient’s pre-transfusion sample testing at the 37ºC IAT phase, they do have the potential to be clinically significant, however, there is usually no requirement for selection of antigen negative donor red blood cells. Instead, red blood cell units that are crossmatch compatible at 37°C IAT phase should be selected for transfusion. See Table 1 for a summary of recommendations for red blood cell transfusion in patients with non-ABO antibodies.
Patients with sickle cell disease
For sickle cell disease patients without antibodies, most guidelines recommend transfusion of Rh and K (KEL1) matched units. For those patients with one or more antibodies (current or historical), complete donor phenotype/genotype matching is often recommended. Typically, this matching includes the Rh, Kell, Kidd and Duffy blood group systems along with the S/s antigens. If a sickle cell patient develops an anti-Lea, Leb, or Leab, then antigen negative units should be provided, along with matching for the full Rh and Kell phenotype. Given the increased risk of hyperhemolysis in patients with sickle cell, this clinical context necessitates full compatibility with the patient’s antigen and antibody profile.
Other disease associations
- Leb is one of the gastric epithelial receptors for Helicobacter pylori.
- Lewis antigens are expressed in renal tissue, especially on distal tubule epithelium and vessel endothelium. Several studies have implicated Lewis antibodies in renal allograft loss in Le(a-b-) recipients, however there is insufficient evidence to include Lewis as a routine histocompatibility marker.
Table 1: Red blood cell transfusion for patients with non-ABO antibodies
| Patient Antibody | Recommendation for red blood cell transfusion* |
|---|---|
| Diego system | |
| Anti-Dia | Red blood cell units crossmatch compatible by IAT at 37°C |
| Anti-Wra | Red blood cell units crossmatch compatible by IAT at 37°C |
| Kell system | |
| Anti-Jsa | Jsa-negative red blood cell units crossmatch compatible by IAT at 37°C |
| Anti-Kpa | Red blood cell units crossmatch compatible by IAT at 37°C |
| Lewis system | |
| Anti-Lea, Anti- Leb, and Anti-Leab | Red blood cell units crossmatch compatible by IAT at 37°C |
| Lutheran system | |
| Anti-Lua | Red blood cell units crossmatch compatible by IAT at 37°C |
| MNS system | |
| Anti-M | Red blood cell units crossmatch compatible by IAT or equivalent using IgG antihuman globulin |
| Rh system | |
| Anti-Cw | Red blood cell units crossmatch compatible by IAT at 37°C |
| Anti-V | V-negative red blood cell units crossmatch compatible by IAT at 37°C |
* Note: Patients with sickle cell disease who develop any one of the antibodies listed here should be provided with antigen-negative red blood cell units for transfusion.
Additional resources
For an introduction to immunohematology and the foundations of blood bank compatibility testing, visit LearnSerology.ca, an online educational resource developed by transfusion medicine specialists in Canada. The curriculum consists of six modules and includes an interactive module for completing an antibody investigation panel.
Suggested citation
Peng S, Meunier D. Lewis System: Anti-Lewis [Internet]. Ottawa: Canadian Blood Services; 2019 Oct 9 [cited YYY MM DD]. Available from: https://profedu.blood.ca/en/transfusion/best-practices/serological-best-practices/lewis-system-anti-lewis
Resources
- Cooling L. Chapter 12: ABO, H, and Lewis Blood Groups and Structurally Related Antigens; The Lewis system. In: Fung M, Grossman B, Hillyer C, Westhoff C, editors. Technical Manual, 18th Ed. Bethesda: AABB; 2014. p. 304-6.
- Reid M, Lomas Francis C and Olsson M. The Blood Group Antigens Facts Book. 3rd Ed. San Diego: Elsevier Science & Technology; 2012. Section II: The blood group systems and antigens; Lewis Blood Group System. p. 347-59.
- Daniels G. Human Blood Groups 3rd ed. Oxford: Wiley-Blackwell; 2013. Chapter 2, ABO, H and Lewis Systems, 2.17: Lewis Antibodies. p. 60-62.
- Milkens C, Berryman J, Cantwell C, et al. for BCSH. Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. Transfus Med. 2012 Dec 06;23(1):1-71 https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-3148.2012.01199.x
- Davis BA, Allard S, Qureshi A, et al. Guidelines on red cell transfusion in sickle cell disease. Part I: principles and laboratory aspects. Br J Haematol. 2016 Nov 07;176(2):145-330. http://www.b-s-h.org.uk/guidelines/guidelines/red-cell-transfusion-in-sickle-cell-disease-part-l/
- The Canadian Haemoglobinopathy Association. Transfusion. In: Consensus Statement on the Care of Patients with Sickle Cell disease in Canada. Version 2.0. Ottawa; 2018. p. 12-20. https://www.canhaem.org/wp-content/uploads/2018/05/Sickle-Cell-Consensus.pdf