Surveillance report 2022
We are pleased to present this annual report describing transmissible blood-borne infection surveillance. High quality and timely surveillance is central to the safety of the blood supply. This includes monitoring of transmissible disease markers that the blood is tested for (including bacteria) and investigation of any reports of possible transfusion transmission, as well as a horizon scan for any new pathogens that may pose a risk.
To request older Surveillance Reports, please contact us through the Feedback form.
Current report - 2023
Surveillance Report 2022
Author: Sheila O'Brien, RN, PhD
Online publication date: September 2023
Executive summary
This annual report describes surveillance of transmissible blood-borne infections and emerging threats of concern. High quality and timely surveillance are central to the safety of the blood supply. This includes monitoring of transmissible disease markers that the blood is tested for and investigation of any reports of possible transfusion transmission, as well as a horizon scan for any new pathogens that may pose a risk. Non-infectious surveillance of aspects of donor health and safety as well as diagnostic services are also included.
Infectious risk monitoring
The most up-to-date tests for pathogens are used to identify infectious donations and prevent their release for patient use. In 2022, transmissible disease rates per 100,000 donations continued to be very low: 0.3 HIV, hepatitis C (HCV) 7.0, hepatitis B (HBV) 7.5, HTLV 1.2 and syphilis 10.7. Selective testing of donors at risk of Chagas disease identified 1 positive donation, and there were 5 donations positive for West Nile Virus (WNV). Residual risk estimates of a potentially infectious donation from a unit of blood are very low at 1 in 19.7 million donations for HIV, 1 in 41.5 million donations for HCV and 1 in 2.9 million donations for HBV. There was a case of probable transfusion transmission of malaria. Lookback and traceback investigations did not identify any other transfusion transmitted infections. Bacterial growth was identified in 158 platelet products. Of 575 potential peripheral blood stem cell or bone marrow donors tested, none were positive for any infectious marker. Of 181 samples from mothers donating stem cells collected from the umbilical cord and placenta (called “cord blood”) after their babies were born, none were positive for any infectious marker.
A new section on the donor re-entry program was included in this report. Since 2014 donors with false reactive results for HIV, HCV or HBV have been eligible to give a sample after 6 months, and if all infectious tests are negative they may re-commence donating. Just under 1,000 donors have been able to donate again and have given over 8,000 donations.
Horizon scanning for emerging pathogens monitors potential threats to safety. Risk of a tick-borne disease, babesiosis, continues to be monitored. The parasite (Babesia microti) that causes babesiosis appears to be in the early stages of becoming established in a few places in Canada, especially in Manitoba. Travelers and former residents from malaria risk areas are temporarily deferred for malaria risk. In addition, a 3 week deferral for any travel outside Canada, continental USA and Europe reduces risk from short term travel related infections such as Zika virus.
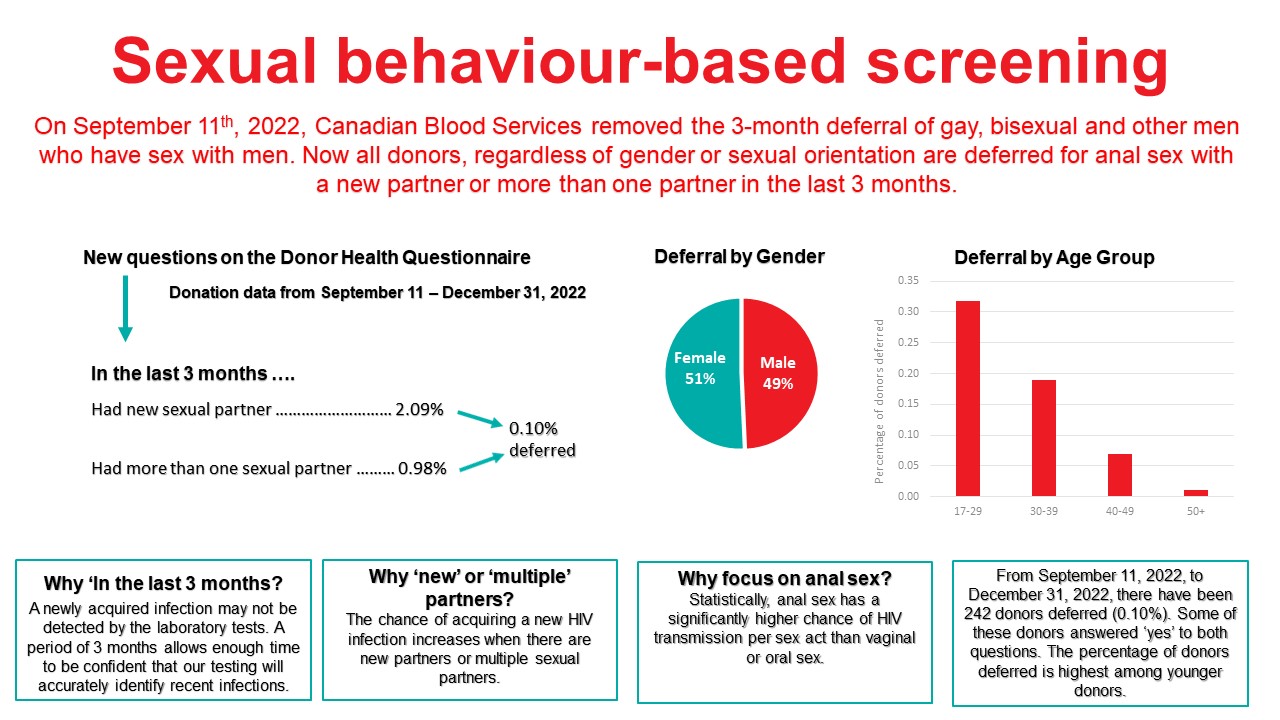
Sexual behaviour-based screening
On September 11, 2022 the 3 month deferral for gay, bisexual, and other men who have sex with men was removed and replaced with deferral based on sexual risk questions that all donors must answer regardless of their gender or sexual orientation. Approximately 0.1% of donors are temporarily deferred using these new sexual behaviour-based questions. No donations were HIV positive.
COVID-19
A novel coronavirus (SARS-CoV-2) was identified in Wuhan, Hubei Province, China in November 2019. On March 11th, 2020, the World Health Organization declared a COVID-19 pandemic. SARS-CoV-2 is not transmissible by blood. Canadian Blood Services has tested over 600,000 blood samples since May 2020 for SARS-CoV-2 antibodies in collaboration with the COVID-19 Immunity Task Force commissioned by the Canadian Federal Government. Seroprevalence to natural infection increased from less about 7% in January 2022 to about 74% in December 2022 when the more infectious variant, Omicron and its sub-variants were dominant. Vaccine related antibodies were found in nearly 100% of donors over 2022, consistent with wide scale vaccination programs. Many donors now have antibodies from both vaccination and infection. These results were important for Public Health officials to plan safety interventions for Canadians.
Diagnostic services laboratories
The Diagnostic Services Laboratories at Canadian Blood Services provide prenatal testing for some pregnant people, including all pregnancies in several provinces (BC, Alberta, and Manitoba), and for some patients with complex transfusion needs. In 2022 1,321 red blood cell antibodies were identified in pregnant patients that may pose a risk of hemolytic disease of the fetus/newborn and in 5,529 patients who may need special matching for transfusion.
Donor demographics
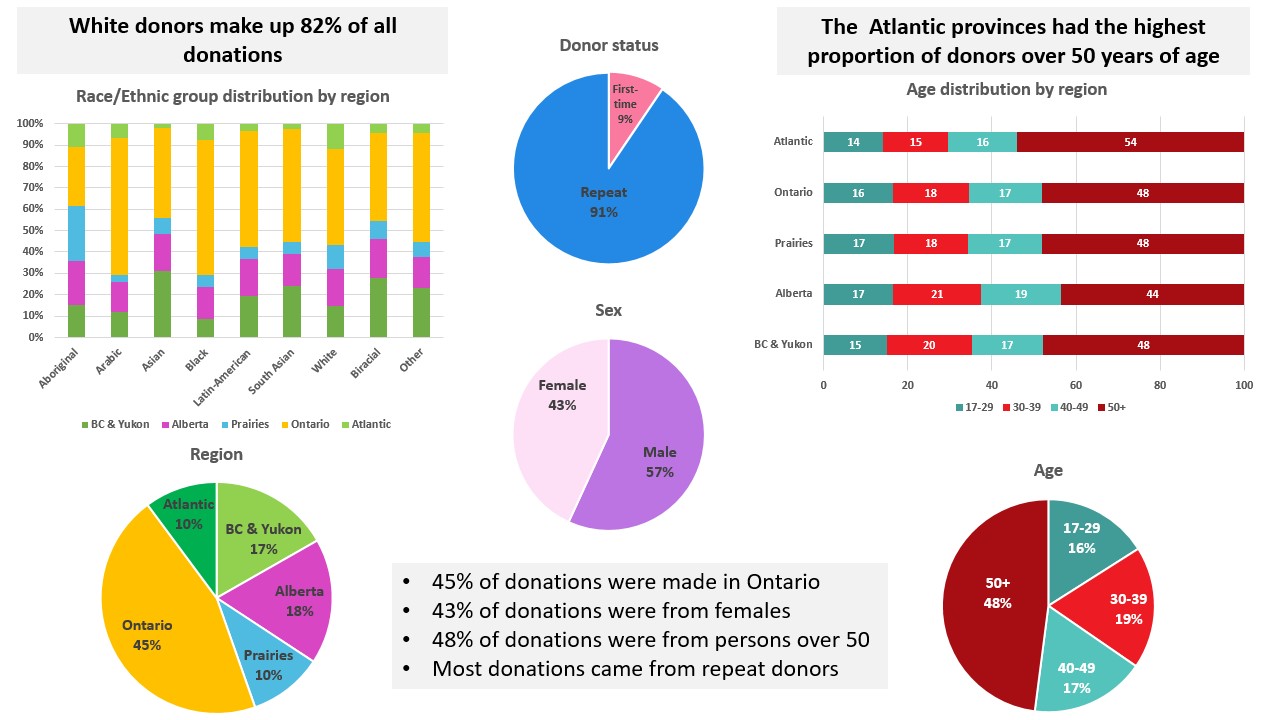
Most donations are repeat donations, a little more than half from males, nearly half (48%) from persons over 50 years of age. Nearly half (45%) of donations are from Ontario. Nearly a fifth of donations are from Black, Indigenous and Racialized donors.
1. Introduction
Safety of the blood supply from pathogens involves a multifaceted approach. Donor education materials on the internet and required reading just before donating explain risk factors for transmissible infections and who should not donate. Before donating blood, everyone must complete a health history questionnaire which includes questions about specific risk factors for transmissible infections. This is followed by an interview with trained staff to decide if the person is eligible to donate blood. All donations are tested for markers of transfusion transmissible agents including HIV, HBV, HCV, human T-cell lymphotropic virus (HTLV) (a rare cause of leukemia) and syphilis. WNV testing is done during the at-risk period of the year (spring, summer and fall) and in at-risk travelers during the winter. In addition, donors at risk of Chagas disease (which is transmitted by the bite of an insect in Latin America) are tested, and platelet products are tested for bacteria. A new coronavirus, SARS-CoV-2, which causes the respiratory infection, COVID-19, emerged in 2020 but it is not transmitted by blood transfusion. Additionally, epidemiologic data on the frequency of antibodies in our donor population over time provided support for public health decision making during the pandemic.
Surveillance includes monitoring of transmissible infection testing in donors, investigation of possible transfusion transmitted infections in recipients and horizon scanning for new, emerging pathogens. Monitoring the safety of donors is also essential. Although surveillance is conducted in "real time" over each year, final verification steps generally impose a short delay in producing a final report. Surveillance also includes monitoring of donor safety. This report describes Canadian Blood Services’ approach to surveillance of transmissible blood-borne infection, infectious threats and donor safety, as well as data for the calendar year of 2022.
2. Blood donor surveillance
The numbers of allogeneic blood donations (whole blood and platelet and plasma apheresis) from first time and repeat donors are shown in Figure 1. The majority of donations are from repeat donors (90.5%). The decreasing trend in the proportion of donations from first time donors from 11.1% in 2019 to 9.8% in 2020 to 8.3% in 2021 appears to be reversing with 9.5% in 2022.
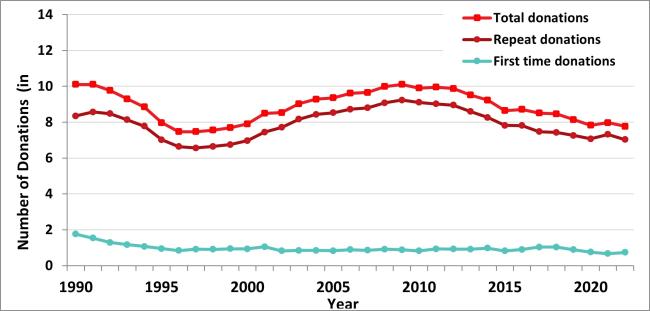
Note: Source plasma donations are not included.
The “Classical” pathogens
Details of screening tests used, and dates of implementation are shown in Appendix I. In Table 1 the numbers of positive donations and the rates of positive tests per 100,000 donations are shown for 2022 by demographic groups. When a transmissible infection is detected, it is most often in a first-time donor as these donors have not been tested previously and may have acquired the infection at any time in their lives. All transmissible infection positive donations occurred in whole blood donations. There were 2 HIV positive donations in 2022. In the past 5 years the number of HIV positive donations has ranged from 0 to 4 per year. The rate per 100,000 donations has decreased for most markers and the rate for repeat donations is extremely low (see Appendix II). The exception is syphilis which has increased from 4.1 per 100,000 donations in 2019 to 9.8 in 2020, 6.7 in 2021 and 10.7 per 100,000 in 2022. Syphilis cases have been increasing in the general population but are not directly comparable because public health cases only include people who had reason to be tested (usually new infections with symptoms) and whereas blood donors include both people unaware of their infection and those who may have been infected in the past. It is unlikely that syphilis could be transmitted by transfusion due to modern blood processing methods.
In April 2021 the serologic testing assay was changed and an increase in HCV positive donations among those previously negative on the old test was observed both in 2021 and again in 2022. Of 6 anti-HCV positive repeat donations one was also NAT reactive and likely a current infection. One had not donated since 1989 before testing was ever in place, one had a negative donation after the change and is likely a new infection. The other 3 had been negative on their last donation using the former assay. The new assay could be detecting low level antibodies from older infections that were resolved and not infectious.
Note: One donation was positive for both HCV and HBV and one for both HCV and syphilis.
All transmissible infection positive donations are destroyed. The main source of risk is when a blood donor acquired the infection too recently to be detected by testing. This is called the “window period” of infection. With current testing the window period is very short. For HIV and HCV an infection would be detected within 1 to 2 weeks of a donor being infected by nucleic acid testing (NAT) and for HBV within one month. The residual risk of infection is the estimated risk of a potentially infectious donation being given during the “window period”. These estimates, shown in Table 2 are based on new infections (positive donations with a prior donation which tested negative within the last 3 years) and include 2019 -2022 data. The risk is currently extremely low, but of course it can never be zero.
Table 1: Confirmed positive donations and prevalence rates per 100,000 donations in 2022
| Characteristic | Number of Donations | Percent of Donations | HIV | HCV | HBV | HTLV | Syphilis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos | Rate | Pos | Rate | Pos | Rate | Pos | Rate | Pos | Rate | |||
| Donor status | ||||||||||||
|
First time |
73,566 | 9.47 | 1 | 1.4 | 48 | 65.3 | 56 | 76.1 | 9 | 12.2 | 51 | 69.3 |
|
Repeat |
702,858 | 90.53 | 1 | 0.1 | 6 | 0.9 | 2 | 0.3 | 0 | - | 32 | 4.6 |
| Sex | ||||||||||||
|
Female |
335,035 | 43.15 | 2 | 0.6 | 17 | 5.1 | 10 | 3.0 | 6 | 1.8 | 19 | 5.7 |
|
Male |
441,389 | 56.85 | 0 | - | 37 | 8.4 | 48 | 10.9 | 3 | 0.7 | 64 | 14.5 |
| Age | ||||||||||||
|
17-29 |
124,260 | 16.00 | 0 | - | 4 | 3.2 | 8 | 6.4 | 0 | - | 14 | 11.3 |
|
30-39 |
144,435 | 18.60 | 0 | - | 11 | 7.6 | 13 | 9.0 | 2 | 1.4 | 23 | 15.9 |
|
40-49 |
135,434 | 17.44 | 2 | 1.5 | 13 | 9.6 | 17 | 12.6 | 2 | 1.5 | 18 | 13.3 |
|
50+ |
372,295 | 47.95 | 0 | - | 26 | 7.0 | 20 | 5.4 | 5 | 1.3 | 28 | 7.5 |
| Total | 776,424 | 100 | 2 | 0.3 | 54 | 7.0 | 58 | 7.5 | 9 | 1.2 | 83 | 10.7 |
Table 2: Estimated residual risk of HIV, HCV and HBV
| HIV | HCV | HBV |
|---|---|---|
| 1 in 19.7 million donations | 1 in 41.5 million donations | 1 in 2.9 million donations |
Risk factors
Risk factor interviews are carried out with donors who test positive for transmissible infections. The main risk factors are shown in Table 3. HIV infections are very rare in donors; therefore, it is difficult to generalize the risk factors. It should be noted that participation is voluntary and therefore there are only data for some donors, and that for many donors no risk factors were identified.
Table 3: Risk factors for infectious disease in blood donors
| Infection | Risk Factor |
|---|---|
| HIV | High risk heterosexual partners Male to male sex |
| HCV |
History of intravenous drug use |
| HBV | Ethnic origin from a higher prevalence country History of living with someone who had hepatitis |
| HTLV | Born overseas (especially Caribbean) History of other sexually transmitted disease History of blood transfusion |
| Syphilis | Previous history of syphilis Male to male sex Sex with an intravenous drug user Born in a higher prevalence country |
Note: Not all donors are available/willing to be interviewed.
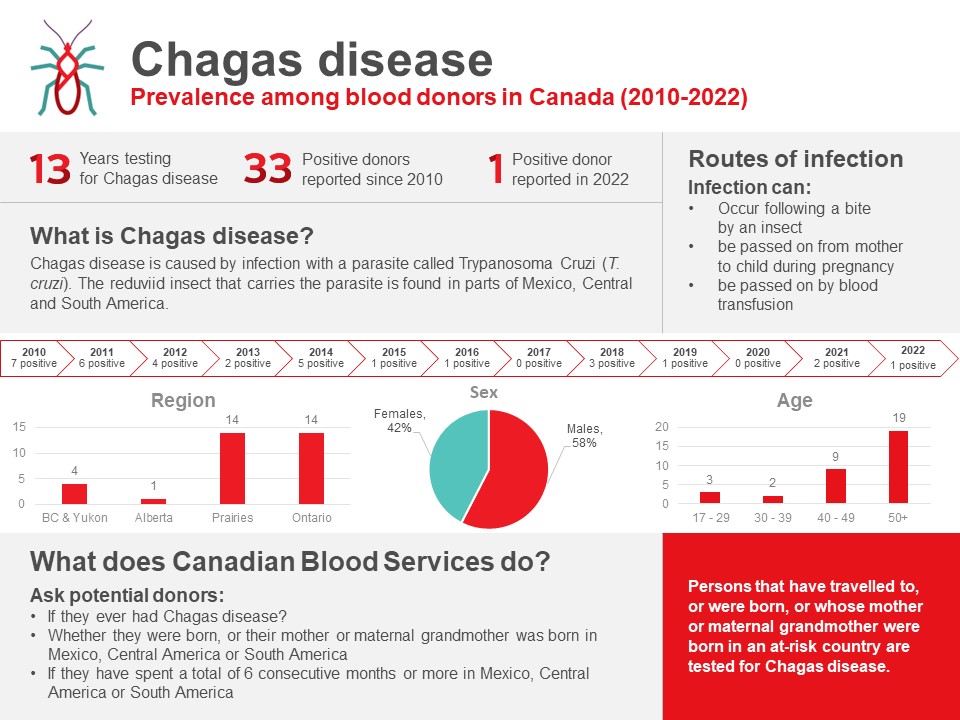
Chagas disease (Trypanosoma cruzi)
Chagas disease is caused by infection with a parasite called Trypanosoma cruzi (T. cruzi). People can become infected with it after being bitten by an insect found in parts of Mexico, Central and South America. The T. cruzi parasite can also be passed on from mother to child during pregnancy and by blood transfusion. Since Canadian Blood Services implemented testing of at-risk donors in 2010, 33 T. cruzi positive donations have been identified (see Figure 2). There was 1 positive donation in 2022 of 7,223 tested.
West Nile virus
West Nile virus is a mosquito-borne virus that has been present in North America since 1999 (in Canada since 2002). Although symptoms can be severe, they are usually mild, and most people are not aware of their infection. During spring, summer and fall, donations are routinely tested in mini-pools of 6 donations. However, to further reduce the risk, an algorithm is applied to identify all donations from areas where West Nile virus is active, and these are tested as single donations. In 2022, 435,337 donations were tested over the spring/summer/fall when all donations were tested, and 5 donations were positive, identified from August 19 to September 13. Four positive donations were from Ontario and 1 from New Brunswick. In 2022 clinical cases were reported in Ontario and Manitoba. With only travelers tested over the winter, 32,145 donations (from Jan 1 to May 29, 2022, and November 27 to December 31, 2022) were tested and none found to be positive.
3. Donor re-entry program
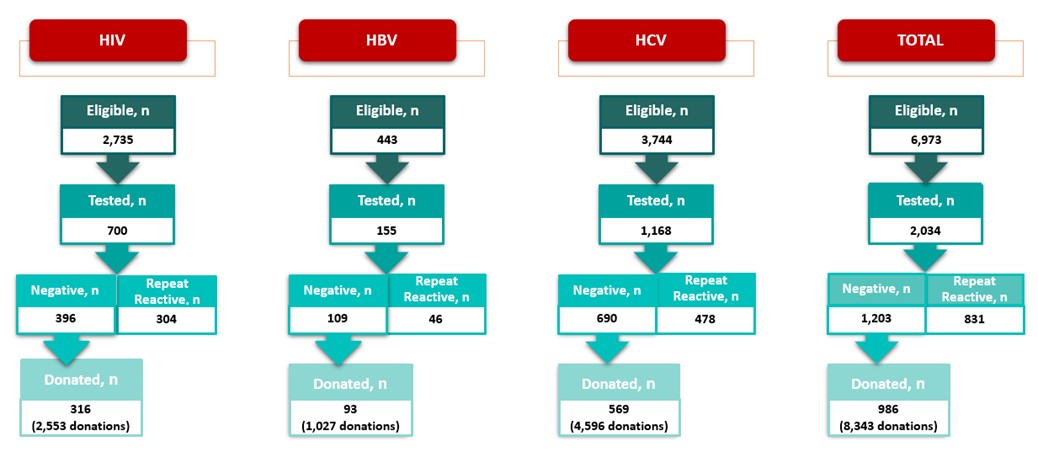
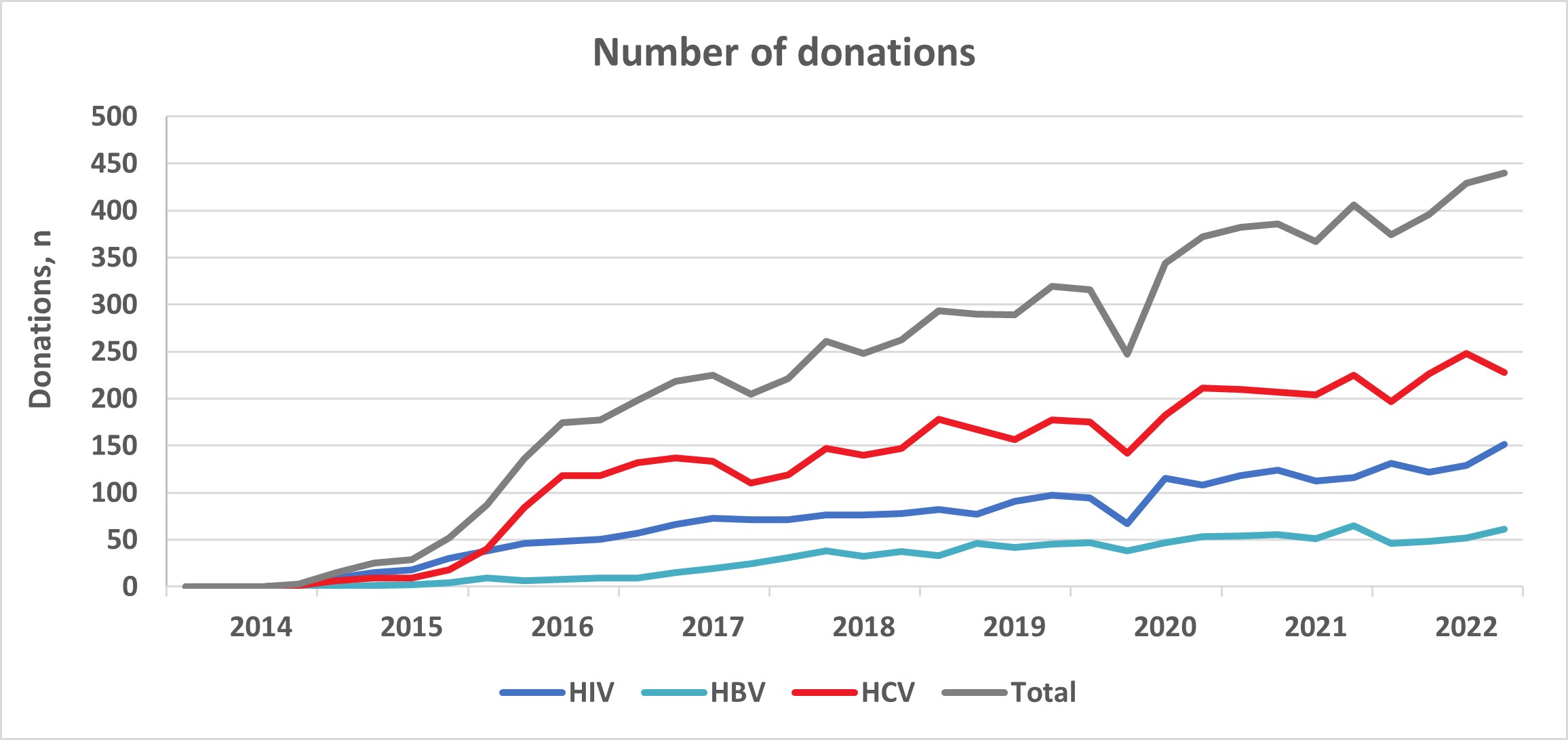
In 2014, Canadian Blood Services established a donor re-entry program for donors who had been indefinitely deferred for HIV, HBV, HCV serological markers that were false positive and false reactive nucleic acid tests for HIV, HCV and HBV. These false positive results occur because very sensitive tests are used for blood screening and sometime react to non-infection related things in the blood. Donors with false positive results are now coded as eligible for donor re-entry and are deferred for 6 months. They are eligible to return any time after 6 months since their last donation to provide an initial specimen-only donation to be re-tested. Donors who are negative for all routinely screened infectious disease markers are then eligible to return to donate blood products. Donors are informed of the donor re-entry program in the donor notification letter that is sent to them following their reactive, but false positive test result. Figure 3 shows the number of donors who were eligible, tested and donated from February 3, 2014, to December 31, 2022, for HIV, HBV and HCV false positive tests and the total. Just under a thousand donors have been able to donate again and have given over 8,000 donations.
However less than 30% of donors eligible for re-entry return to provide an initial specimens-only donation. Between half and three quarters of donors are eligible to donate after their specimen is tested depending on the marker and most return to donate (82% overall). Figure 4 shows the increasing trend in the number of donations per quarter from 2014 to 2022 from donors who re-entered the donor pool.
4. Surveillance for emerging pathogens
A horizon scan of potential blood borne infections in the general community ensures rapid revision of donor policies to maintain safety. Even before a new infectious disease is reported in Canada, we are aware of emerging infectious agents by monitoring outbreaks in other parts of the world. As international travel becomes more frequent post-COVID-19 pandemic it is important to be vigilant as infections can rapidly enter from other countries. To ensure that potential risks are identified, Canadian Blood Services needs to be connected with the latest infectious disease information at all times. Canadian Blood Services’ medical and scientific staff participate in public health and infectious disease professional organizations and monitor web sites and journals where new information is posted. When appropriate the Alliance of Blood Operators (ABO) Risk Based Decision Making Framework can be used to facilitate policy decision making. This ensures that relevant assessments including infectious risk to recipients, operational impact of strategies, stakeholder input and health economics are considered. A range of infectious agents that could emerge as a threat within Canada are being monitored.
Coronavirus disease 2019 (COVID-19)
SARS-CoV-2 is a novel coronavirus first identified in Wuhan, Hubei province of China in late 2019. It is responsible for a severe respiratory illness known as the 2019 coronavirus disease (COVID-19). Some people become extremely ill and can die from complications, while others experience mild symptoms and may not be aware of their infection. On March 11th, 2020, the World Health Organization declared COVID-19 to be a pandemic. Since then, over 4.6 million Canadians have received a COVID-19 diagnosis and over 50,000 people have died.
SARS-CoV-2 (COVID-19) seroprevalence
Early in the pandemic with less than 500 confirmed cases across Canada (March 23rd, 2020), strict physical distancing measures were implemented in most provinces. As a result, the first wave of the epidemic peaked by the end of April and plateaued in July and August 2020. Multiple waves fueled by new variants followed. In December of 2021 cases of the Omicron variant appeared. Vaccination of Canadians began in December 2020 and was rolled out over 2021 (2 shots). Starting in November 2021, some Canadians became eligible for a third dose, and in 2022 some people could also have a fourth shot. About 89% of adults have received at least the primary series (2 shots).
Public health reported cases do not show the true infection rate because some infections will not cause illness, others may not be severe enough for people to seek testing, and testing may be disproportionately directed to outbreaks. Importantly, when the Omicron variant arrived in late 2022 public health testing was overwhelmed by large numbers of cases and limited testing to certain groups such as high-risk individuals. Over 2022 spikes in infection activity were identified with wastewater surveillance, but only seroprevalence could quantify the proportion of people infected. Testing for SARS-CoV-2 antibodies is important to understand what proportion of the population have already been infected (the seroprevalence), what proportion have vaccine related antibodies and to monitor infection over the course of the pandemic. These data are used to improve mathematical models to predict the course of infection, understand population immunity and inform public health policy. In partnership with the COVID-19 Immunity Task Force, Canadian Blood Services is testing residual blood for SARS-CoV-2 antibodies. As of December 31, 2022, over 600,000 samples from across Canada have been tested for SARS-CoV-2 antibodies, of which about 374,000 were in 2022. Two types of antibodies were tested for: antibodies to nucleocapsid due to infection, and antibodies to spike protein due to vaccination or infection. With the roll out of the primary vaccine series in early 2021 antibodies to the spike protein were mainly caused by vaccination and increased to 90% in June 2021. Seroprevalence due to natural infections (nucleocapsid antibodies) remained low up to December of 2021 at about 7% but with the advance of Omicron and its subvariants seroprevalence increased to about 74% by December 2022. The continued near 100% of donors with spike protein antibodies reflected both infection and vaccination antibodies. Figure 5 shows the changes from April 2020 to December 2022.
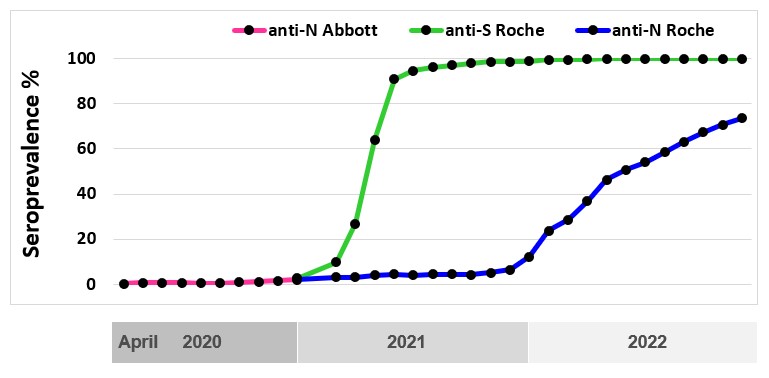
Worldwide, blood services have leveraged their operational capacity to inform public health. SARS-CoV-2 seroprevalence remained low in Canada but there were significant variations by regions, racialized individuals, and socioeconomic factors. Canadian Blood Services is committed to continuing to play a pivotal role in helping authorities evaluate public health policies, monitor disparities and track antibody concentrations as long as needed.
Babesiosis
Babesiosis comes from the bite of the black-legged tick (Ixodes scapularis) which can transmit the parasite B. microti. Usually it causes mild flu-like symptoms, and many people are not even aware that they have had it. However, it can also be transmitted by blood transfusion, and infection in blood recipients can result in severe illness or death. To date babesiosis cases in the general population have been reported mainly in the Northeastern and Upper Midwest parts of the United States where more than 1,500 cases per year are reported. Cumulatively more than 200 infections in the United States are believed to have been acquired from a transfusion. In Canada there has been one case of transfusion transmitted babesiosis in 1998 from a donor who had travelled to the Northeast US. In Canada the parasite is found in small numbers of ticks. A 2013 study at Canadian Blood Services and Héma-Québec tested 13,993 blood donations and none were positive. In 2013 one non-donor infected from a tick bite in Canada was reported. Ongoing public health surveillance of ticks suggests no increase in risk, but a donor study was carried out in 2018 involving more donors. One of 50,752 samples tested from southerly areas across Canada was positive by B. microti nucleic acid testing (NAT, donated in Manitoba and indicating an active infection) and of 14,758 samples tested for antibody to B. microti, 4 in Southwestern Ontario were positive (but negative by NAT, indicating likely resolved infection at some time in the past). In 2019 a donor developed illness after donating and was diagnosed with B. microti infection, likely infected in southern Manitoba. No recipients were infected from the donation. The estimated risk of a clinically relevant infection being transmitted from a blood transfusion in Canada is very low at 0.08 per year (0 – 0.38) per year, or about 1 in 12.5 years. The ABO Risk Based Decision Making Framework was used to evaluate possible risk mitigation strategies. Further studies are planned to follow possible expansion of babesia species in Canada.
Travel related infections
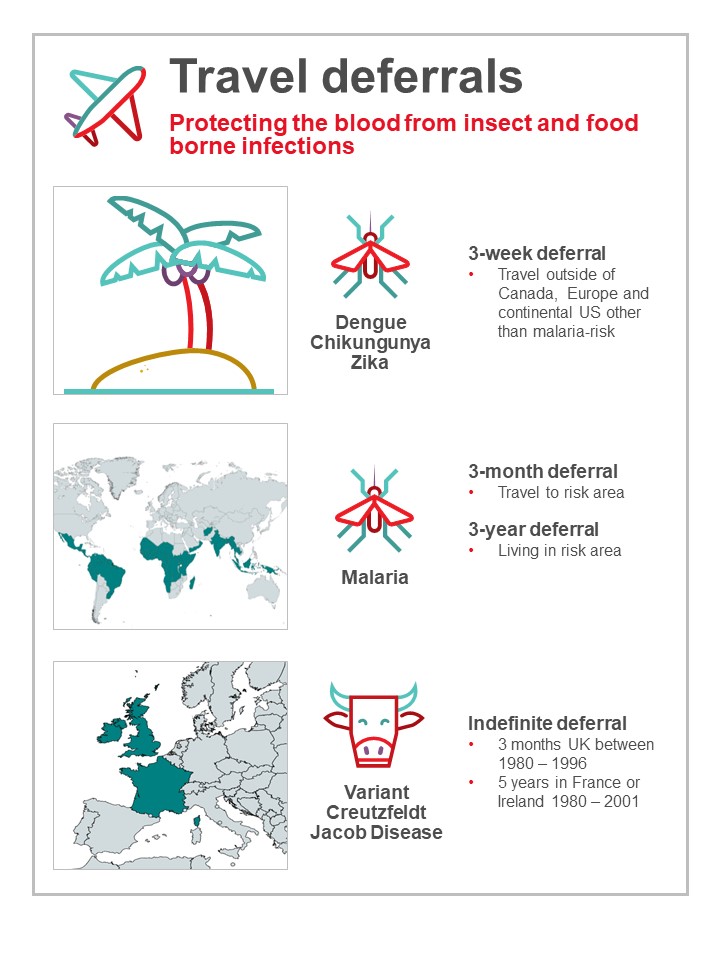
Donors who travel may return with infections that could be transmitted by blood (see Figure 6). Most are only at risk for a period of time after returning until the infection is eliminated from the donor’s bloodstream. Malaria risk is present in parts of the Caribbean, Mexico, Central and South America, Asia, and Africa. Donors are deferred after travel to risk areas for 3 months, enough time to develop symptoms. Former residents of endemic areas are deferred for 3 years because there is a chance they may be infected for a longer time period without symptoms. Other tropical mosquito-borne infections such as dengue virus have long been present in sunny destinations frequented by Canadians, but in recent years there have been outbreaks of others such as Chikungunya virus and Zika virus not previously seen in the Caribbean, Mexico, Central and South America. Risk to the blood supply was determined to be very low based on quantitative risk assessment. However, to address future travel risks, since 2016 Canadian Blood Services defers all donors who have travelled anywhere outside of Canada, continental USA or Europe for 3 weeks after travel. By 2020 COVID-19 was circulating in Canada but travel-related infections were also of concern, especially new variants that may be rare in Canada. Although COVID-19 is not transfusion transmissible, in order to protect staff and other donors, a 2 week deferral for all travel outside of Canada was implemented including travel to the US and Europe. This reverted back to the original 3 week deferral in May 2022, with no deferral for travel to continental US or Europe.
Residency or cumulative travel considered a risk for variant-Creutzfeldt Jakob disease (vCJD) results in permanent deferral. vCJD is acquired from eating infected meat (Bovine Spongiform Encephalitis, or BSE, known as “Mad Cow Disease”). Donors are deferred for time spent in France, Ireland or the UK while infected meat may have been available. However, deferrals for time spent in Western Europe (excluding Ireland and France) and Saudi Arabia were lifted in 2022 due to very low risk in those countries.
5. Bacteria
Bacteria in blood products usually come from the skin of donors during their blood donation, although occasionally they may come from the donor’s bloodstream. The number of bacteria is usually very low, but because platelet products are stored at room temperature, bacteria can multiply to reach high concentrations and then pose a serious risk to the recipient. Canadian Blood Services tests all apheresis and pooled platelet products for bacteria using the BACT/ALERT System in which a sample from the product is inoculated into an aerobic (presence of oxygen) culture bottle and an anaerobic (absence of oxygen) culture bottle and monitored for growth for 7 days. If any bacterial growth is detected in the culture bottles, the product is not issued if it is still in inventory at Canadian Blood Services. If it has been sent to a hospital, it is recalled and returned to Canadian Blood Services from the hospital blood bank (i.e., has not been transfused or discarded). In 2022, 106,983 platelet products (17,652 apheresis and 89,721 pooled products) were tested, of which 108 apheresis and 382 pooled products had initial positive results for bacterial growth in the culture bottles. From these, 8 and 96 cultures were confirmed as true bacterial contaminations, for apheresis and pooled products, respectively. In addition, 8 apheresis and 46 pooled products with initial positive results were not confirmed as they were issued and/or transfused. This represents 158 products in total (14.8 per 10,000) with a chance of bacterial contamination with current testing, including both true positives and suspected positives.
6. Lookback/traceback
All cases of potential transfusion transmission of infections are investigated. The Lookback / Traceback Program is notified when a donor tests positive for a transmissible infection, or if the donor reports a transfusion transmissible infection after donating (even if it is not one that would normally be tested for). A Lookback investigation is initiated when previous or historical donations are identified, and hospitals are asked to contact the recipients of these donations to arrange testing. A traceback is initiated when a recipient is found to have a transmissible infection and transfusion was confirmed and it is queried as to whether their infection could have been from their blood transfusion. Hospitals provide a list of all blood products that the recipient received, and Canadian Blood Services attempts to contact the donors of these products to arrange testing unless current test results are available.
In 2022, there were 18 Lookback cases that required investigation: 13 HCV, 1 HIV, 3 HBV, 1 malaria. 10 of these were from donors who had a positive transmissible disease marker and 8 were from external testing or public health notification. Of these, 14 cases were closed (all recipients that could be contacted were tested). The remaining 4 cases were still open. There were 11 cases from previous years closed in 2022. One closed case was associated with transfusion transmission of malaria (transfusion in 2022, linked to the traceback case noted below).
There were 14 Traceback cases that required investigation received from external sources in 2022 (8 HCV, 5 HBV, 1 malaria). Of these, 10 cases were closed (all donors that could be contacted were tested), and 4 remain open. There were 12 cases from previous years closed in 2022. One closed case was associated with transfusion transmission of malaria (transfusion in 2022, linked to the positive lookback case noted above).
7. Blood stem cells
Blood stem cells can multiply to renew themselves; the new cells develop into blood cells such as red cells, white cells and platelets. In adults, they are found mainly in the marrow of large bones, with a few cells in the bloodstream. The cord blood of newborn babies, taken from the umbilical cord and placenta after the delivery of a healthy baby, is also very rich in stem cells. Blood stem cells can therefore be obtained from the bone marrow, from circulating blood (called peripheral blood stem cells) or from the umbilical cord (cord blood) after a baby is born. Blood stem cells are very important in treating various diseases such as leukemia, lymphoma and multiple myeloma. Canadian Blood Services has a coordinated national stem cell program which includes adult registrants and banked cord blood units. Infectious disease testing for all stem cell products includes the same markers tested for whole blood donations.
Canadian Blood Services’ stem cell registry
Canadian Blood Services Stem Cell Registry is a registry of Canadians who have volunteered to donate either bone marrow or peripheral blood stem cells should a recipient need it at some time in the future. Potential registrants complete a questionnaire which includes risk factors for transmissible infections and are tested for their Human Leukocyte Antigen (HLA) profile. In 2022 there were about 440,000 registrants in the registry. In total, 575 registrants were identified as potential matches for recipients and had additional testing. There were no infectious disease positive results.
Canadian Blood Services’ cord blood bank
Canadian Blood Services’ Cord Blood Bank collected cord blood at four sites in Canada in 2022 (Ottawa, Brampton, Edmonton, and Vancouver). Participating mothers at these hospitals who volunteer to donate their baby’s cord blood complete a questionnaire about medical conditions that could be passed on to a recipient, as well as risk factors for transmissible infections. If the donation is suitable for transplantation (i.e., has enough stem cells) with negative results for all infections, the cells are frozen and stored until a recipient needs them. In 2022, there were 181 blood samples from mothers tested and none had infectious disease positive results.
8. Transitioning from male-to-male sex deferral to sexual behaviour based screening
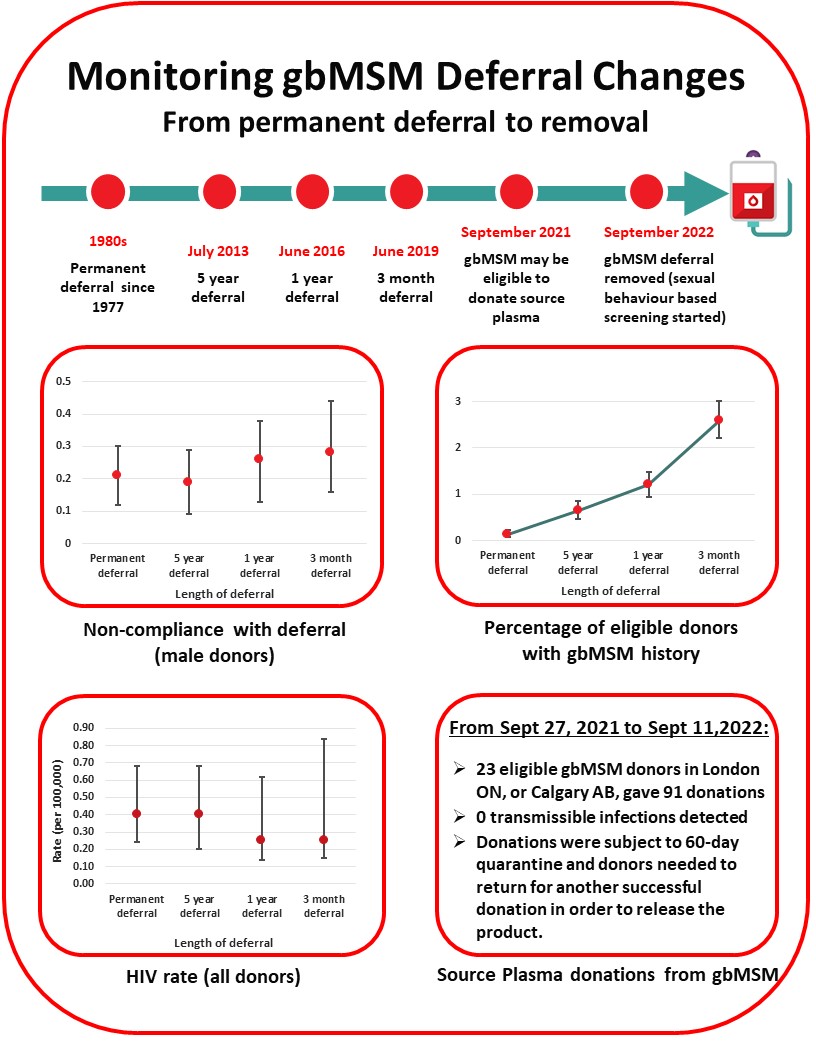
In the 1980’s men who had sex with another man even once since 1977 were not eligible to donate blood to reduce the risk of HIV transmission. With much improved donor testing and surveillance for emerging pathogens the deferral period has been gradually reduced, moving to 5 years in 2013, to 1 year in 2016 and to 3 months in 2019. HIV rates did not increase following any of these changes (see Figure 7). Anonymous donor compliance surveys showed that shortening the deferral period allowed more gay, bisexual and other men who have sex with men (gbMSM) to donate blood and donor compliance was not adversely affected (see Figure 7). With the 3-month deferral in place the risk of releasing an HIV infectious unit for transfusion was very low at 1 in 19.9 million donations (1 in 2.7 million – 1 in 1,668 million).
gbMSM Research Grants
Canadian Blood Services is committed to ongoing revision of the gbMSM deferral policy. In 2017, Canadian Blood Services and Héma-Québec launched a competitive grant program to allocate funding provided by Health Canada to Canadian researchers to address the challenges in removing deferral criteria and ensuring the safety of the blood supply would be maintained. Fifteen research projects were funded by this grant program. In 2020, a second competitive grant program with funding provided by Health Canada focused on research related to plasma donation for gbMSM and four research projects were funded. For more information see: https://blood.ca/en/research/our-funded-research-projects?combine=msm.
Plasma donation
In September 2021, as an interim step sexually active gbMSM became eligible to donate source plasma at two donation centres (London, Ontario and Calgary, Alberta) if they had not had a new sexual partner in the last three months, the donor and their partner had only had sex with each other in the last three months, and the donor met all the other criteria for donation. Plasma donated by gbMSM was stored until the donor returned to donate again 60 days after their donation and had another negative test. The product was then released. The plasma was used to make plasma protein products, and as part of the processing, pathogen inactivation technology inactivates a broad spectrum of pathogens. On September 11th, 2022, the plasma program for gbMSM transitioned to sexual behaviour based screening for all donors (see below) and the quarantine step ceased.
From September 27th, 2021, to September 10th 2022, there were 91 plasma donations from sexually active gbMSM who met the criteria. All donations tested negative for infectious disease markers. More information about plasma eligibility can be found here: Am I eligible to donate plasma? | Blood.ca
Sexual behaviour-based screening
Many countries have switched from lifetime to shorter time deferrals, and recently a number of countries have removed their time-based deferrals in favour of individual behaviour-based screening. The UK and the Netherlands removed their time-based deferral in 2021, and France removed their time-based deferral in 2022, all with different risk reducing donor eligibility criteria. The US currently has a 3-month deferral in place, but new FDA Guidance will allow removal of the time based deferral and implementation of behaviour-based screening.
On September 11th 2022, Canadian Blood Services removed the 3 month deferral for men having had sex with another man and implemented two sexual behaviour-based screening questions that all donors must answer irrespective of sexual orientation or gender (See Figure 8). As of December 31st 2022 0.1% of donors presenting to donate were temporarily deferred based on these sexual risks (see Figure 7). Deferred donors tended to be younger and were slightly more common in females than males. No donations tested positive for HIV during this period.
9. Donor safety
Donor reactions
Canadian Blood Services takes many precautions to make sure that giving blood is safe for donors. These include a health screening questionnaire and a hemoglobin fingerstick screen, as well as providing refreshments and monitoring the donor after donating. Most donors do not have any problems during or after their donation, but it is important to keep track of any incidents that happen so that donor care can be improved. Definitions of reactions are shown in Table 4.
Table 4: Reaction and definitions
| Reaction | Definition |
|---|---|
| Vasovagal
Moderate Severe |
Donor loses consciousness (faint reactions)
Unconscious less than 60 seconds and no complications Unconscious more than 60 seconds or complications |
| Major Cardiovascular Event | Chest pain or heart attack within 24 hours of blood donation, may or may not be related to donation |
| Re-bleed | The phlebotomy site starts to bleed after donation |
| Nerve Irritation | Needle irritation or injury of a nerve during phlebotomy. Usually described as sharpshooting pain, arm tingling or numbness |
| Inflammation/Infection | Redness or infection at the needle site, usually seen several days after donating |
| Local Allergic Reaction | Rash from skin cleaning solution or dressing, with raised vesicles on the skin |
| Arm Pain | Usually due to blood pressure cuff, tourniquet or arm position |
| Bruise/Hematoma | Temporary dark colour of the skin due to blood leakage from blood vessel at time of phlebotomy |
| Arterial Puncture | Needle inserted in an artery instead of a vein |
Reaction rates per 10,000 whole blood donations in 2022 are shown in Figure 9 with those in 2021 for comparison. Moderate and severe vasovagal reactions were not significantly different (p>0.05). People more likely to experience a reaction are first-time donors, young donors (17-25 years old) and female donors. The reaction reporting system is oriented towards capturing moderate and severe reactions. Most reactions are mild, such as feeling faint or bruising at the needle site, but these are only recorded if mentioned by the donor at some point after donation. A further breakdown of fainting reactions (both moderate and severe) in whole blood donors by sex and donation history is provided in Table 5.
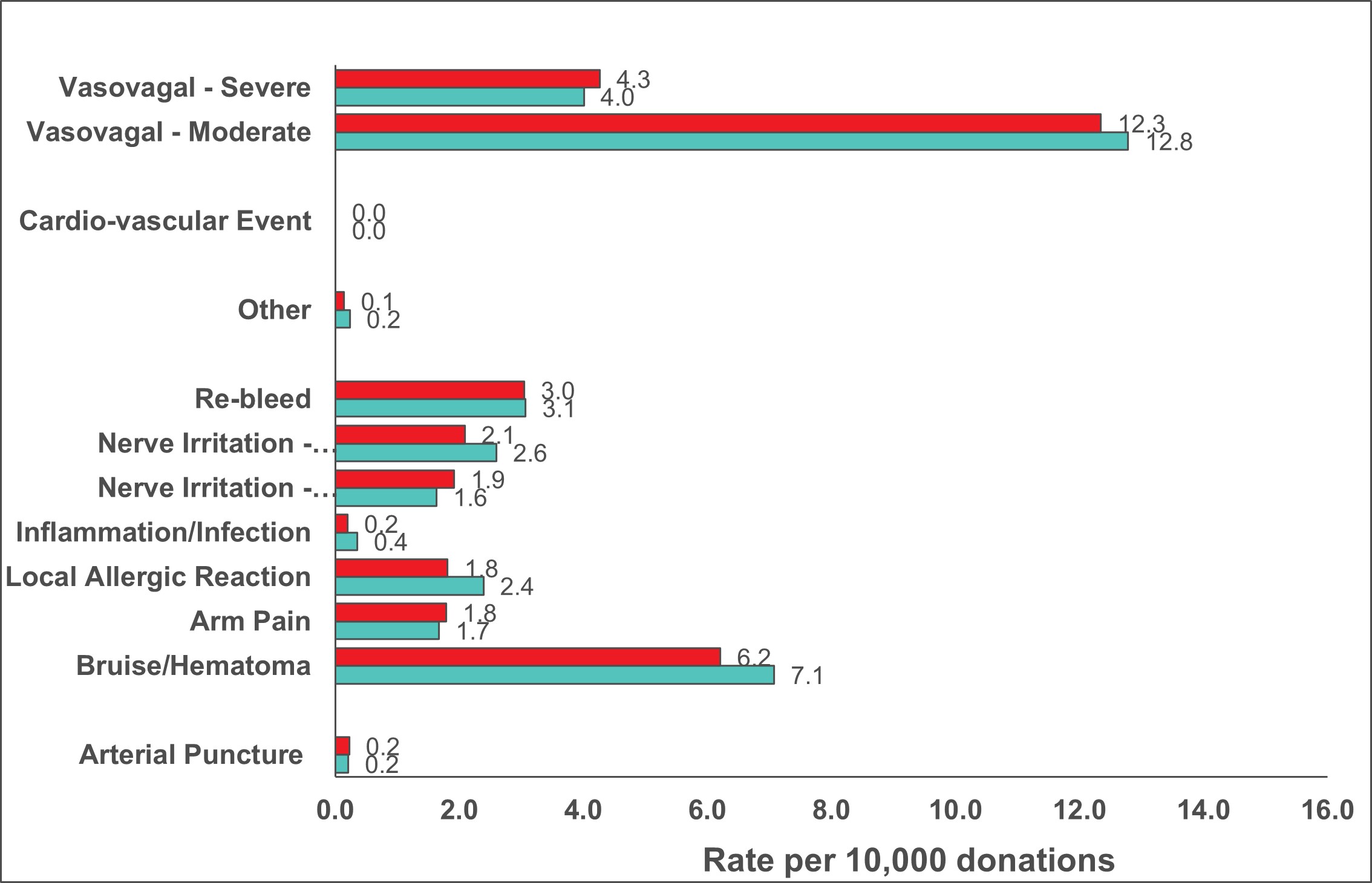
Table 5: 2022 Fainting (vasovagal) reactions (per 10,000 collections)
| Donation Status | Moderate & Severe (all) | Associated with injury | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| First Time | 57.0 | 82.9 | 2.4 | 3.6 |
| Repeat | 5.9 | 18.5 | 0.4 | 1.4 |
|
Injuries were generally not severe such as bruises and cuts from falling *all comparisons (male vs female, first time vs repeat) are statistically significant among moderate & severe reactions combined (p<0.01). For reactions associated with injury all were significant (p<0.01), except for male first time and female first time donors (p=0.4). |
||||
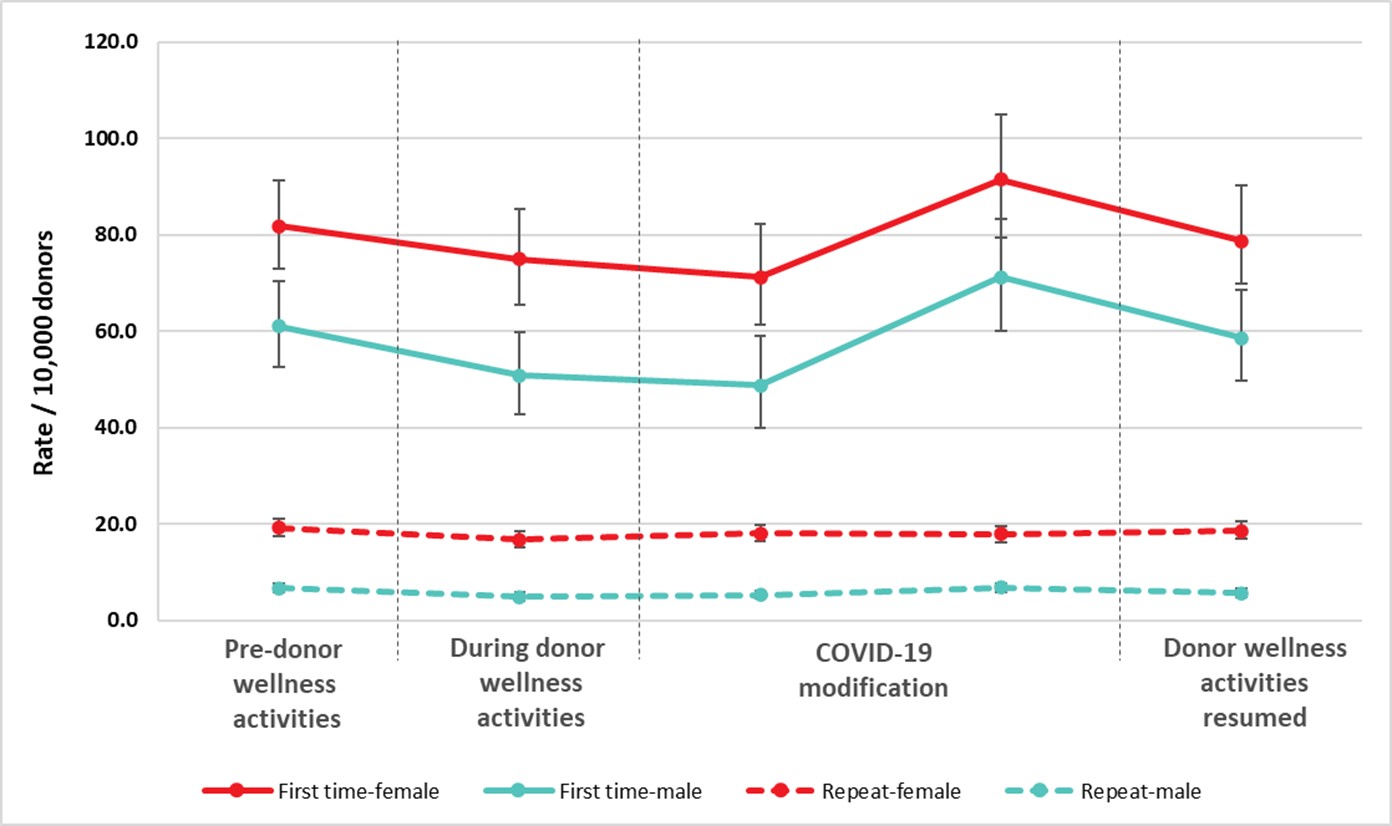
Pre-donation water and salty snacks were put in place in 2019 to reduce vasovagal (faint) reactions. Donor instructions to carry out muscle tension exercises while donating continued to be provided. These can also reduce the risk of a vasovagal reaction. Collectively the pre-donation water and salty snacks and muscle tension exercises were part of the Donor Wellness initiative. During the pandemic collection sites provided post-donation refreshments as always but asked donors to have them outside of the clinic. Collection sites stopped providing salty snacks and water before donating, although donors were encouraged to have their own before coming to the clinic. Between May and June 2022, collection sites phased back in pre-donation salty snack and water. Figure 10 shows the reaction rates before putting in place the donor wellness activities, while they were in place, after stopping the pre-donation water and salty snacks and during the time donor wellness activities were resumed. Initially there was a downward trend in vasovagal reactions for all groups associated with donor wellness activities. After taking into account gender and donation status, the vasovagal reaction rates were lower during the wellness activities and early after the water and salty snacks were stopped, but later when they were still stopped and even after they resumed the rates returned to the pre-donor wellness level (p<0.05). The uptake of wellness activities once resumed may have been less than when first implemented.
Donor hemoglobin and iron
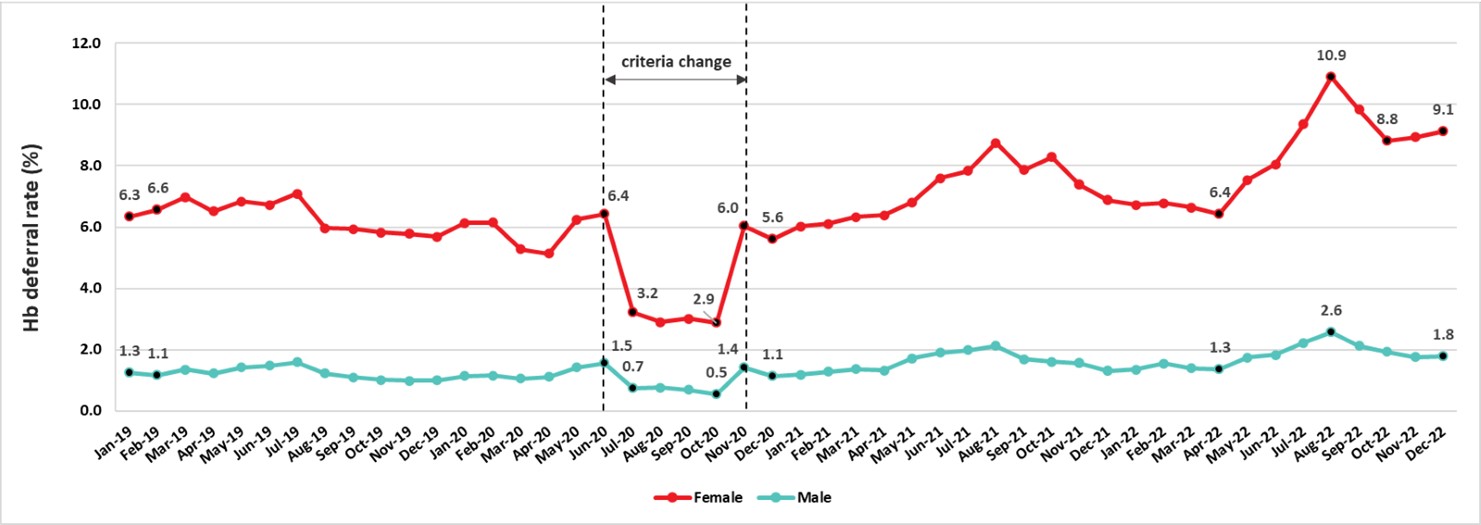
The most common reason for a donor deferral at the collection site is a failed hemoglobin fingerstick screen. Low hemoglobin is often related to low iron stores. Iron is needed to make hemoglobin which carries oxygen in red blood cells. Studies at Canadian Blood Services showed that iron stores are often lower in females and are further reduced by frequent donation in both females and males. Males with borderline hemoglobin are also more likely to have lower iron stores. To reduce the chance of developing iron deficiency, the hemoglobin concentration required for males was increased from 125 g/L to 130 and the minimum wait time between whole blood donations for females was increased from 56 days to 84 days in 2017. This longer interdonation period allows females more time to build back their iron stores and return to their baseline hemoglobin levels. To reduce the chance of blood shortages if fewer people donated during the pandemic, the minimum hemoglobin was dropped from July 2020 to October 2020 to 120 g/L for females and 125 g/L for males. As shown in Figure 11 the deferrals decreased from 7% to 3.2% (p<0.01) for females and from 1.5% to 0.8% (p<0.01) for males during that period. However, after October the minimum hemoglobin switched back to the original values and deferrals increased, especially in female donors where they were higher than before the pandemic and remained so. A change in instruments to measure hemoglobin in 2022 may have influenced higher deferrals.
Information about iron and the safety of blood donation can be found at www.blood.ca as well as in the ‘What you must know to give blood’ pamphlet provided to all donors prior to every donation.
10. Donor demographics
The proportions of whole blood donations by demographic variables are shown in Figure 14 and Figure 15. All blood donors are asked a voluntary question about their racial/ethnic group, which assists the laboratory in selecting donor samples for additional testing for rare blood groups that are more frequent in certain populations. Over 95% of donors answer this question. While most donations are given by donors who self-identify as White (82%), the ethnicity of Black, Indigenous and Racialized donors varies by geographic region. Figure 14 shows the percentage of donations from donors of each ethnic group in each region. In October, 2022 the choice of racial/ethnic categories was expanded, updated, and harmonized between stem cell and whole blood donors, Most donations are repeat donations, a little more than half from males, nearly half (48%) from persons over 50 years of age, and nearly half (45%) are from Ontario.
11. Diagnostic services
The Diagnostics Services laboratories provide patient testing, mainly for pregnant patients (Perinatal Laboratories) and patients receiving blood transfusions (Crossmatch/Reference Laboratories). Some Canadian Blood Services Diagnostic Services Laboratories provide all of these services.
| Maternal | 98.38% |
| Paternal | 0.73% |
| Cord | 0.89% |
| 164,072 samples tested |
Perinatal laboratories
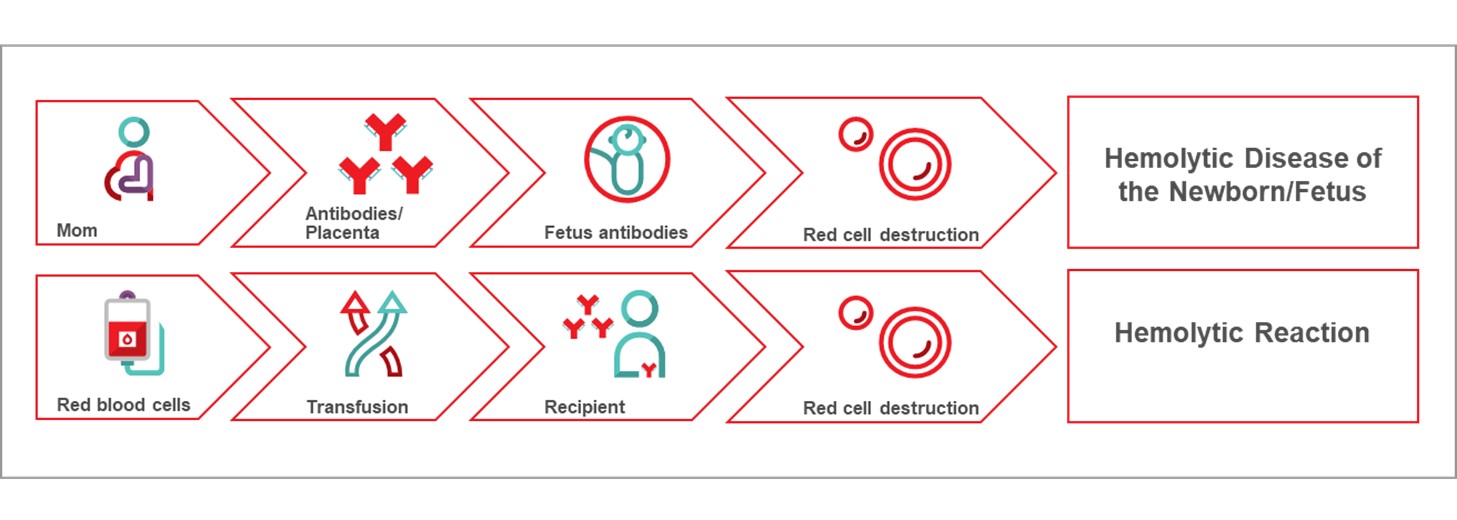
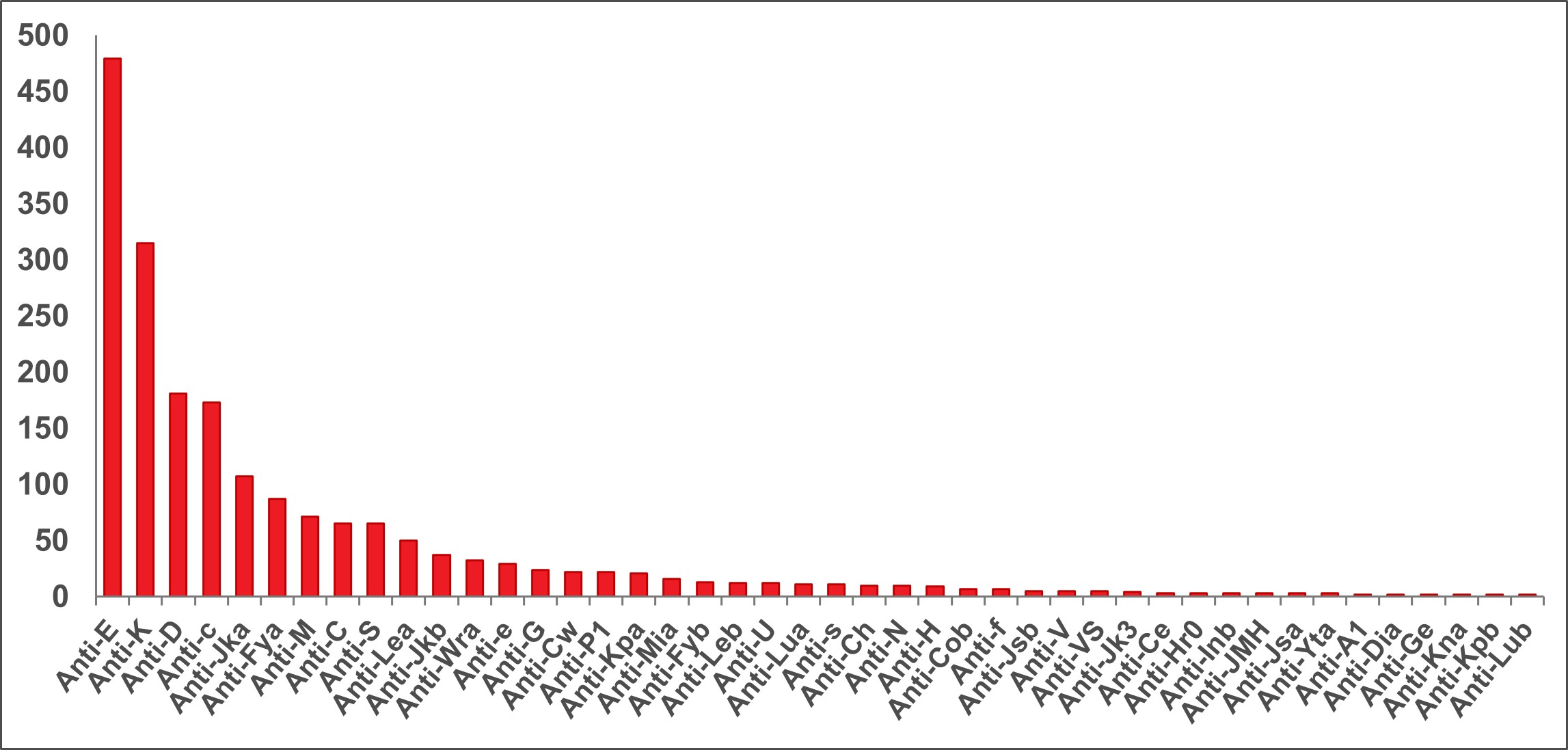
The Perinatal Laboratories provide testing of pregnant patients for blood group and antibodies to red blood cells. Some antibodies can cause hemolytic disease of the fetus/newborn. The goal is to (1) identify Rh negative pregnant patients and recommend treatment to prevent developing anti-D antibodies, and (2) identify pregnant patients with risky antibodies to monitor their pregnancy and treat as needed. Testing of fathers, newborns and fetuses is also sometimes done. In 2022, 1,321 pregnant (perinatal) patients had clinically significant antibodies; the most common ones that could put their baby at risk are shown in Figure 14.
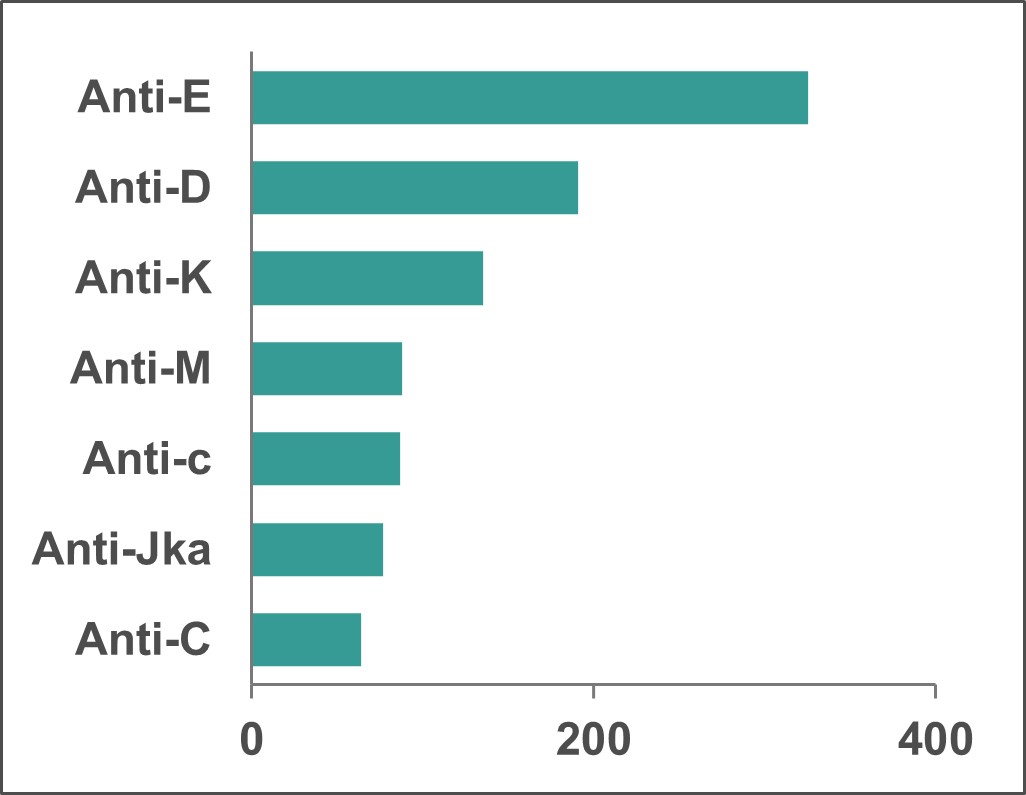
*Note: Anti M is rarely clinically significant.
Crossmatch/reference laboratories
The Reference Laboratories provide testing of patients for blood groups which must match the donor blood to be transfused. They also carry out antibody investigations for patients who may have unusual red blood cell antibodies and need special matching of blood for transfusion. Figure 15 shows the frequency of different antibodies in pre-transfusion patients (5,529 patients). Patients who have rare antibodies/antigens may be difficult to match for transfusion. The National Platelet Immunohematology Platelet Reference Lab in Winnipeg, Manitoba assists health care providers manage thrombocytopenic patients by Human Leukocyte Antigen (HLA) and Human Platelet Antigen (HPA) typing and investigation for HLA and HPA. HLA and HPA typed platelets are available for transfusion support of these patients.
For more details see https://blood.ca/en/hospital-services/laboratory-services/surveys
References
Donor Screening
Vesnaver E, Goldman M, O’Brien SF MacPherson P, Butler-Foster T, Lapierre D, Otis J, Devine DV, Germain M, Rosser A, et al. Barriers and enablers to source plasma donation by gay, bisexual and other men who have sex with men under revised eligibility criteria: protocol for a multiple stakeholder feasibility study. Health Res Policy Sys 2021;18:131.
O’Brien SF, Goldman M, Robillard P, Osmond L, Myhal G, Roy E. Donor screening question alternatives to men who have sex with men time deferral: Potential impact on donor deferral and discomfort. Transfusion 2021;61:94-101.
Caffrey N, Goldman M, Osmond L, Yi QL, Fan W, O’Brien SF. HIV incidence and compliance with deferral criteria over three progressively shorter time deferrals for men who have sex with men in Canada. Transfusion 2022;62:125-134.
Caffrey N, Goldman M, Lewin A, Osmond L, O’Brien SF. Behaviour-based screening questions and potential donation loss using the “for the assessment of individualized risk” screening criteria: A Canadian perspective. Transfus Med 2022;32:422-427.
Saeed S, Goldman M, Uzicanin S, O’Brien SF. Evaluation of a Pre-exposure Prophylaxis (PrEP)/ Post Exposure Prophylaxis (PEP) Deferral Policy among Blood Donors Transfusion 2021;61:1684-1689.
Germain M, Gregoire Y, Custer BS, Goldman M, et al. An international comparison of HIV prevalence and incidence in blood donor and general population: a BEST Collaborative study. Vox Sang 2021;61:2530-2537.
Goldman M. How do I think about blood donor eligibility criteria for medical conditions? Transfusion 2021;61:2530-2537.
Miller O, Caffrey N, O’Brien SF, Goldman M. Evolving policies for donors with diabetes: The Canadian experience. Vox Sang 2022;117:1415-1419.
Quee F, SF O’Brien, Prinsze et al. Whole blood donor return rates after deferral for tattooing of body piercing-Survey across blood donation services: the BEST collaborative study. Vox Sang 2022;117:1085-1089.
Jacquot C, Tiberghien P, van den Hurk K, Ziman A, Shaz B, Apelseth TO, Goldman M, The BEST Collaborative. Blood donor eligibility for medical conditions: A BEST collaborative study. Vox Sang 2022;117:929-936.
Residual Risk
O’Brien SF, Gregoire Y, Pillonel J, Steele WR, Custer B, Davison K, Germain M, Lewin A, Seed CR. HIV residual risk in Canada under a three-month deferral for men who have sex with men. Vox Sang 2020;115:133-139.
Caffrey N, Goldman M, Lewin A, Gregoire Y, Yi QL, O’Brien SF. Removing the men who have sex with men blood donation deferral: Informing risk models using Canadian public health surveillance data. Transfus Clin Biol 2022;29:198-204.
Babesia microti
O’Brien SF, Drews SJ, Yi QL, Bloch EM, Ogden NH, Koffi JK, Lindsay LR, Gregoire Y, Delage G. Risk of transfusion-transmitted Babesia microti in Canada. Transfusion 2021;61:2958-2968.
Drews SJ, Van Caeseele P, Bullard J, Lindsay LR, Gaziano T, Zeller MP, Lane D, Ndao M, Allen VG, Boggild AK, O’Brien SF, Marko D, Musuka C, Almiski M, Bigham M. Babesia microti in a Canadian blood donor and lookback in a red blood cell recipient. Vox Sang 2022;117:438-441.
Hepatitis E
Fearon MA, O’Brien SF, Delage G, Scalia V, Bernier F, Bigham M, Weger S, Prabhu S, Andonov A. Hepatitis E in Canadian blood donors. Transfusion 2017;57:1420-1425.
Delage G, Fearon M, Gregoire Y, Hogema BM, Custer B, Scalia V, Hawes G, Bernier F, Nguyen ML, Stramer S. Hepatitis E virus infection in blood donors and risks to patients in the United States and Canada. Trans Med Rev 2019;33:139-145.
Hepatitis B
O’Brien SF, Reedman CN, Osiowy C, Bolotin S, Yi QL, Lourenco L, Lewin A, Binka M, Caffrey N, Drews SJ. Hepatitis B blood donor screening data: An under-recognized resource for Canadian public health surveillance. Viruses 2023;15:409.
Bacteria
Ramirez-Arcos S, Evand S, McIntyre T, Pang C, Yi QL, DiFranco C, Goldman M. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-2928.
Malaria
O’Brien SF, Ward S, Gallian P, Fabra C, Pillonel J, Davison K, Seed CR, Delage G, Steele WR, Leiby DA Malaria blood safety policy in five non-endemic countries: a retrospective comparison through the lens of the ABO risk-based decision-making framework. Blood Transfus 2019;17:94-102.
HTLV
O’Brien SF, Yi QL, Goldman M, Gregoire Y, Delage G. Human T-cell lymphotropic virus: A simulation model to estimate residual risk with universal leukoreduction and testing strategies in Canada. Vox Sang 2018;113:750-759.
Iron deficiency
Goldman M, Uzicanin S, Osmond L, Yi QL, Scalia V, O’Brien SF. Two year follow-up of donors in a large national study of ferritin testing. Transfusion 2018;25:2868-2873.
Goldman M, Yi QL, Steed T, O’Brien SF. Changes in minimum hemoglobin and interdonation interval: impact on donor hemoglobin and donation frequency. Transfusion 2019;59:1734-1741.
Chasse M, Tinmouth A, Goldman M et al. Evaluating the clinical effect of female donors of child-bearing age on maternal and neonatal outcomes: A cohort study. Transfusion Medicine Reviews, 2020;24:117-123.
Blake JT, O’Brien SF, Goldman M. The impact of ferritin testing on blood availability in Canada. Vox Sang 2022;117:17-26.
Donor wellness
Goldman M, Germain M, Gregoire Y, Vassallo RR, Kamel H, Bravo M, O’Brien SF. Safety of blood donation by individuals over age 70 and their contribution to the blood supply in five developed countries: a BEST Collaborative group study. Transfusion 2019; 59:1267-1272.
Goldman M, Uzicanin S, Marquis-Boyle L, O’Brien SF. Implementation of measures to reduce vasovagal reactions: Donor participation and results. Transfusion 2021;61:1764-1771.
Public health
O’Brien SF, Drews SJ, Lewin A, Russell A, Davison K, Goldman M. How do we decide how representative our donors are for public health surveillance? Transfusion 2022;62:2431-2437.
O’Brien SF, Drews SJ, Lewin A, Osiowy C, Drebot MA, Renaud C. Canadian blood suppliers: An expanded role in public health surveillance? Can Comm Dis Rep 2022;48:124-130.
O’Brien SF, Goldman M, Drews SJ. An expanded role for blood donor emerging pathogen surveillance CMAJ 2023;195:E16.
COVID-19
Stanworth SJ, New HV, Apelseth TO, Goldman M et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. The Lancet Haematology 2020;7:757-764
Drews SJ, Hu Q, Samson R, Abe KT, Rathod B, Colwill K, Gingras AC, Yi QL, O’Brien SF. SARS-CoV-2 virus-like particle neutralization capacity in blood donors depends on serological profile and donor-declared SARS-CoV-2 vaccination history. Microbiol Spectr 2022;10:e0226221.
Lin YJ, Evans DH, Robbins NF, Orjuela G, Hu Q, Samson R, Abe KT, Rathod B, Colwill K, Gingras AC, Tuite A, Yi QL, O’Brien SF, Drews SJ. Utilization of Abbott SARS-CoV-2 IgG II quant assay to identify high-titer anti-SARS-CoV-2 neutralizing plasma against wild-type and variant SARS-CoV-2 viruses. Microbiol Spectr 2022;10:e0281122.
Saeed S, Drews SJ, Pambrun C, Yi QL, Osmond L, O’Brien SF. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion 2021;61:862-872.
Reedman CN, Drews SJ, Yi QL, Pambrun C, O’Brien SF. Changing patterns of SARS-CoV-2 seroprevalence among Canadian blood donors during the vaccine era. Microbiol Spectr 2022;10:e0033922.
O’Brien SF, Caffrey N, Yi QL, Bolotin S, Janjua NZ, Binka M, Thanh QC, Stein DR, Lang A, Colquhoun A, Pambrun C, Reedman NC, Drews SJ. Cross-Canada variability in blood donor SARS-CoV-2 seroprevalence by social determinants of health. Microbiol Spectr 2023;14:e0335622
O’Brien SF, Caffrey N, Yi QL, Pambrun C, Drews SJ. SARS-CoV-2 seroprevalence among Canadian blood donors: The advance of Omicron. Viruses 2022;14:2336.
Climate change and infectious risks
Drews SJ, Wendel S, Leiby DA, Tonnetti L, Ushiro-Lumb I, O’Brien SF Climate change and parasitic risk to the blood supply. Transfusion 2023;63:638-645.
Appendix I: Implementation dates of testing
| Marker | Implementation Date* | ||
|---|---|---|---|
| 1 | Syphilis | 1949 | |
| 2 |
HBV (Hepatitis B Virus) |
||
|
|
HBsAg |
1972 |
|
|
|
Anti-HBc |
2005 |
|
|
|
HBV NAT |
2011 | |
| 3 |
HIV (Human Immunodeficiency Virus) |
||
| Anti-HIV-1 EIA (enzyme-linked immunosorbent assay) | 1985 | ||
|
Anti-HIV-1/2 EIA |
1992 | ||
| HIV-1 p24 antigen | 1996 (discontinued in 2003, resumed in 2021) | ||
| HIV-1 NAT | 2001 | ||
| Anti-HIV-1/2 (including HIV-1 subtype O) EIA | 2003 | ||
| 4 |
HTLV (Human T-Lymphotropic Virus) |
||
| Anti-HTLV-I | 1990 | ||
| Anti-HTLV-I/II | 1998 | ||
| 5 |
HCV (Hepatitis C Virus) |
||
| Anti-HCV EIA/ELISA | 1990 | ||
| HCV NAT | 1999 | ||
| 6 |
WNV (West Nile Virus) |
||
| WNV NAT | 2003 | ||
| 7 | Chagas’ disease (Trypanosoma cruzi) selective testing | 2010 | |
| 8 | Bacteria | ||
| BacT Alert | 2004 | ||
| BacT Alert modified for 7 day platelets | 2017 | ||
|
*These are the dates that testing for the marker began. Tests have been upgraded as new versions of the test became available. |
|||
Appendix II: Rate of HIV, HCV, HBV, HTLV and syphilis in first-time and repeat donations
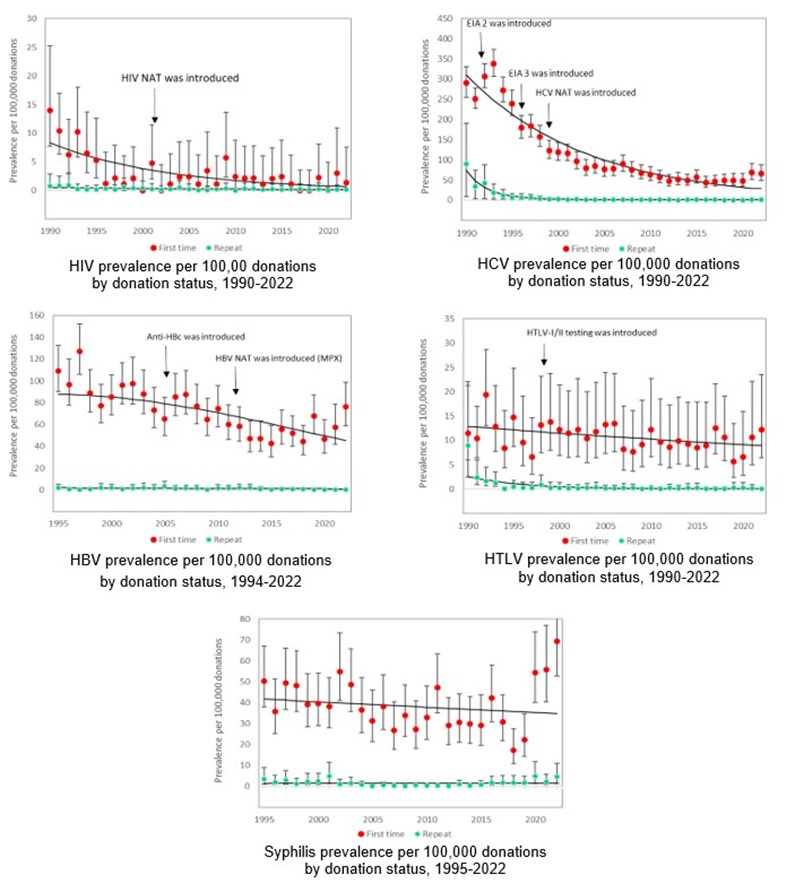
Note that these graphs have different scales on the y-axis.